Intravenous Product Packaging Market Overview
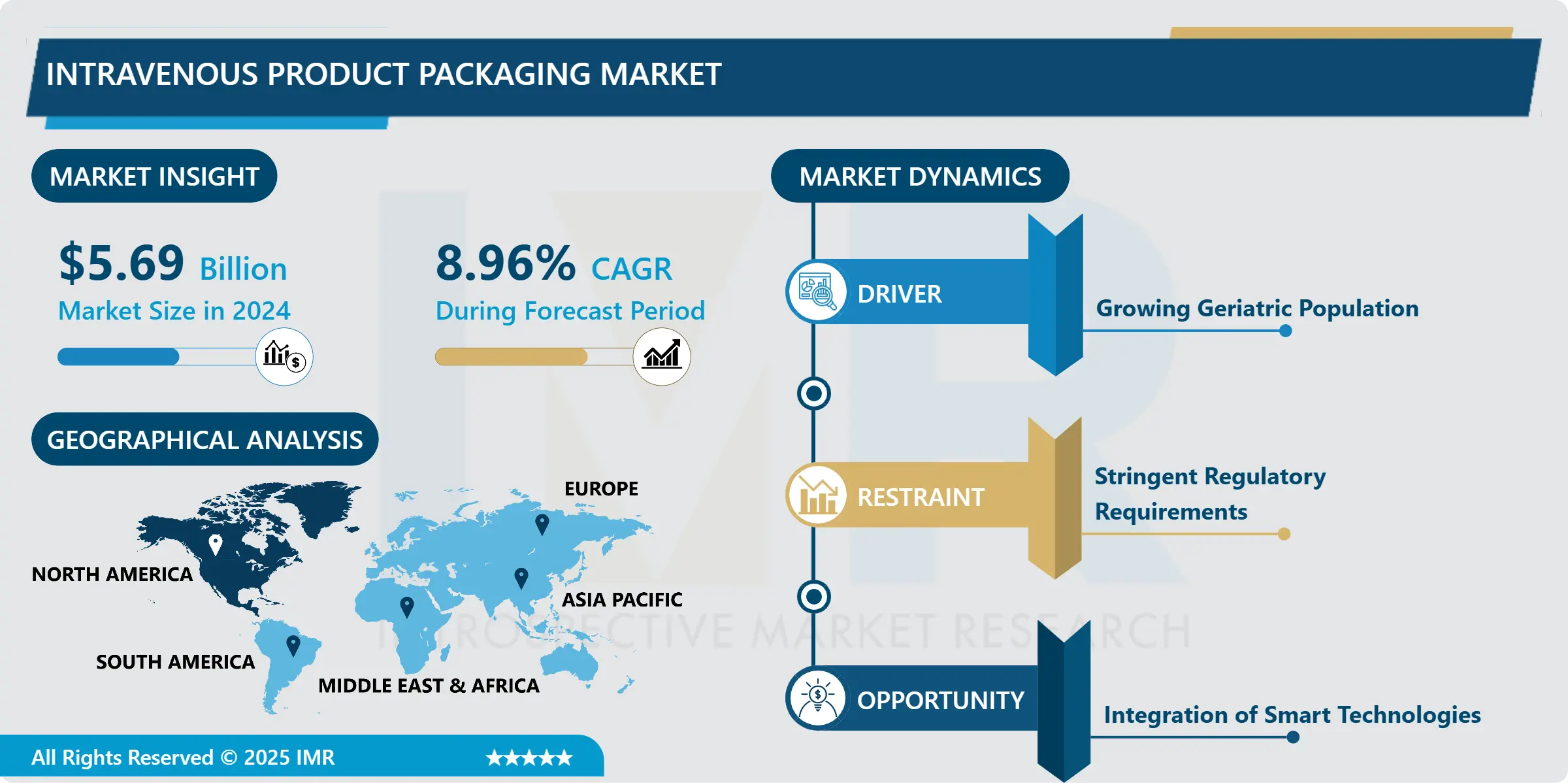
Intravenous Product Packaging Market Size Was Valued at USD 5.69 Billion in 2024 and is Projected to Reach USD 14.62 Billion by 2035, Growing at a CAGR of 8.96% From 2025-2035.
Intravenous product packaging is a medical device used to store and protect intravenous (IV) products, which are medications or fluids administered directly into a vein. It must meet several requirements, including sterility, compatibility, permeability, integrity, transparency, and ease of use. IV products must be sterile to prevent contamination, and the packaging must be compatible with the IV product to prevent any adverse reactions.
The packaging must be impermeable to bacteria and contaminants while allowing the IV product to pass. It must withstand shipping, handling, and storage without leaking or breaking. Transparency is essential for visual inspection and ease of use, even for healthcare professionals wearing gloves. Common types of IV product packaging include IV bags, cannulas, syringes, and infusion pumps. Intravenous product packaging is crucial in the healthcare industry, as it ensures the safety and effectiveness of IV products for patients.
The primary benefit of IV product packaging is to protect the sterility and integrity of the contents. This is essential to prevent contamination and ensure that patients receive the intended medication or fluid. IV product packaging also plays an important role in drug delivery, allowing for precise administration of medications and fluids. IV product packaging offers advantages over other methods of medication delivery, such as fast delivery, controlled delivery, and direct delivery. Fast delivery ensures quick absorption into the bloodstream, while controlled delivery allows for precise dosage and timing. Direct delivery bypasses the digestive system, ensuring full absorption.
Intravenous Product Packaging Market Analysis:
Growing Geriatric Population
-
The increasing elderly population worldwide has led to a surge in demand for healthcare services, including intravenous therapies, due to chronic illnesses requiring frequent medical interventions. This has intensified the need for efficient and secure packaging solutions for intravenous products.
-
The ageing population, with a higher prevalence of conditions like cardiovascular diseases, diabetes, and cancer, demands targeted medical interventions. This has increased demand for intravenous therapies, driving the growth of the IV product packaging market. Packaging ensures the safety, sterility, and efficacy of intravenous products, and as healthcare providers cater to the unique needs of the geriatric population, demand for advanced packaging solutions has surged.
-
The geriatric population faces mobility and medication self-administration challenges, leading to increased reliance on healthcare facilities for intravenous treatments. This shift has increased demand for user-friendly packaging, catering to healthcare professionals and patients. Manufacturers in the intravenous product packaging market are focusing on innovations to enhance ease of use, reduce contamination risks, and ensure medication integrity.
-
The IV product packaging market is experiencing robust expansion due to the growing geriatric population and the increasing demand for intravenous therapies. The market is poised for sustained growth, driven by the imperative to deliver safe, effective, and convenient intravenous treatments to this demographic, highlighting the need for advanced packaging solutions in the evolving healthcare landscape.
Integration of Smart Technologies
-
The healthcare industry is embracing smart solutions to improve patient care, streamline processes, and enhance efficiency, particularly in intravenous product packaging, where the incorporation of smart technologies opens new opportunities for innovation and value addition.
-
Smart technologies like RFID and the IoT are revolutionizing intravenous product management and monitoring. They enable real-time tracking and monitoring from manufacturing to healthcare institutions, ensuring supply chain integrity and reducing errors and tampering. This enhances the safety and quality of intravenous therapies, reducing the risk of tampering.
-
Smart technologies are revolutionizing medication management in IV packaging. They enable the monitoring of medication temperature and integrity during storage and transportation, ensuring optimal conditions for healthcare providers and patients. This transparency and traceability are especially crucial in intravenous therapies, where the efficacy of medications is closely linked to their handling and storage.
-
Smart packaging provides real-time data on patient adherence and treatment progress through smartphone apps or web interfaces. This enhances patient experience and contributes to personalized healthcare delivery by providing valuable information about administered intravenous therapies, improving the overall patient experience. The intravenous product packaging market is poised for significant growth due to the integration of smart technologies like RFID and IoT. These solutions not only improve the safety and efficiency of intravenous therapies but also provide data-driven insights and improved patient outcomes. As the healthcare industry embraces digital transformation, the IV product packaging market will benefit from this innovation.
Intravenous Product Packaging Market Segment Analysis:
Intravenous Product Packaging Market Segmented on the basis of Type, Material, and Application
By Type, IV bags segment is expected to dominate the market during the forecast period
-
The widespread adoption of intravenous therapies across diverse medical disciplines, ranging from critical care to routine medical procedures, has led to a consistent and high demand for IV bags. These bags provide a versatile and convenient solution for administering fluids, medications, and nutrients directly into the bloodstream
-
Material technology advancements in manufacturing processes have significantly impacted the development of IV bags in healthcare. These bags offer enhanced features like compatibility with medications, improved durability, and advanced safety measures. Their popularity among healthcare professionals is driven by their cost-effectiveness, ease of use, and reduced risk of contamination. The flexibility and scalability of IV bags make them a preferred choice in hospital settings and home healthcare environments, ensuring a steady demand in the broader intravenous product packaging market.
Intravenous Product Packaging Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
-
The healthcare industry in North America is thriving due to advanced facilities, research institutions, and a robust regulatory framework. The widespread adoption of intravenous therapies and the ageing population, coupled with chronic diseases, have increased the demand for efficient and secure IV product packaging solutions, driving the growth of the IV product packaging market in the region.
-
North America is at the forefront of IV product packaging developments due to key market players, innovation culture, and early adoption of advanced technologies. The region's commitment to patient safety, product quality, and regulatory compliance reinforces its dominance. Strategic collaborations between healthcare institutions, pharmaceutical companies, and packaging manufacturers contribute to the strength of the North American market, setting the standard for best practices and advancements in healthcare delivery.
Key Players Covered in Intravenous Product Packaging Market:
- Baxter (U.S.)
- Dupont(U.S.)
- Minigrip (U.S.)
- Smith Medical (U.S.)
- BD (U.S.)
- Cardinal Health (U.S.)
- Sippex IV bag (France)
- B. Braun Medicals (Germany)
- Neotec Medical Industries (Singapore)
- SSY Group Limited (Hong Kong)
- Nipro (Japan)
- Technoflex (Japan)
- Terumo Medical Corporation (Japan)
- Forlong Medical Co., Ltd. (China)
- Amcor Plc (Australia)
- MRK Healthcare Private Limited (India), and Other Major Players
Key Industry Developments in the Intravenous Product Packaging Market:
-
In August 2023, Amcor, a global leader in developing and producing responsible packaging solutions, announced it had agreed to acquire Phoenix Flexibles, expanding Amcor’s capacity in the high-growth Indian market. Amcor currently has four flexible packaging plants in India. The business has delivered double-digit organic sales growth per year over the last three years, significantly outpacing growth in the underlying market, and is also investing to double its local footprint in the pharmaceutical and medical packaging categories.
|
Intravenous Product Packaging Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2035 |
|
Historical Data: |
2018 to 2023 |
Market Size in 2024: |
USD 5.69 Bn. |
|
Forecast Period 2025-35 CAGR: |
8.96% |
Market Size in 2035: |
USD 14.62 Bn. |
|
Segments Covered: |
By Type |
|
|
|
By Material |
|
||
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Intravenous Product Packaging Market by Type (2018-2035)
4.1 Intravenous Product Packaging Market Snapshot and Growth Engine
4.2 Market Overview
4.3 IV Bags
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Cannula
4.5 Extension Sets
4.6 Infusion Pumps
4.7 Stopcocks
Chapter 5: Intravenous Product Packaging Market by Material (2018-2035)
5.1 Intravenous Product Packaging Market Snapshot and Growth Engine
5.2 Market Overview
5.3 PVC
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Non-PVC
5.5 Polyolefin
5.6 Polypropylene
Chapter 6: Intravenous Product Packaging Market by Application (2018-2035)
6.1 Intravenous Product Packaging Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Hospitals
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Clinics
6.5 Ambulatory
6.6 Surgical Centers
6.7 Home Care
6.8 Others
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Intravenous Product Packaging Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 3M COMPANY (US)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 ASPEN LASER SYSTEMS LLC (US)
7.4 BEIJING TOPSKY CENTURY HOLDING COLTD (CHINA)
7.5 BHARAT ELECTRONICS LTD. (INDIA)
7.6 BLUEYE AUSTRALIA (BLUEYE TACTICAL) (AUSTRALIA)
7.7 BOLLE SAFETY (FRANCE)
7.8 COFRA SRL (ITALY)
7.9 COLE-PARMER INSTRUMENT COMPANY LLC (US)
7.10 EALING CORPORATION (US)
7.11 ELBIT SYSTEMS LTD (ISRAEL)
7.12 ELVEX CORPORATION (US)
7.13 ESS (US)
7.14 GENTEX CORPORATION (US)
7.15 GLOBAL LASER LTD (UK)
7.16 HAGER & WERKEN GMBH & CO. KG (GERMANY)
7.17 HILCO VISION (US)
7.18 HONEYWELL INTERNATIONAL (US)
7.19 KENTEK CORPORATION (US)
7.20 LASERGLOW TECHNOLOGIES (CANADA)
7.21 METAMATERIAL TECHNOLOGIES (CANADA)
7.22 NOIR LASER COMPANY LLC (US)
7.23 PHILLIPS SAFETY PRODUCTS (US)
7.24 RADIANS INC. (US)
7.25 THORLABS INC. (US)
7.26 UNIVET S.R.L. (ITALY)
Chapter 8: Global Intravenous Product Packaging Market By Region
8.1 Overview
8.2. North America Intravenous Product Packaging Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size by Type
8.2.4.1 IV Bags
8.2.4.2 Cannula
8.2.4.3 Extension Sets
8.2.4.4 Infusion Pumps
8.2.4.5 Stopcocks
8.2.5 Historic and Forecasted Market Size by Material
8.2.5.1 PVC
8.2.5.2 Non-PVC
8.2.5.3 Polyolefin
8.2.5.4 Polypropylene
8.2.6 Historic and Forecasted Market Size by Application
8.2.6.1 Hospitals
8.2.6.2 Clinics
8.2.6.3 Ambulatory
8.2.6.4 Surgical Centers
8.2.6.5 Home Care
8.2.6.6 Others
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Intravenous Product Packaging Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size by Type
8.3.4.1 IV Bags
8.3.4.2 Cannula
8.3.4.3 Extension Sets
8.3.4.4 Infusion Pumps
8.3.4.5 Stopcocks
8.3.5 Historic and Forecasted Market Size by Material
8.3.5.1 PVC
8.3.5.2 Non-PVC
8.3.5.3 Polyolefin
8.3.5.4 Polypropylene
8.3.6 Historic and Forecasted Market Size by Application
8.3.6.1 Hospitals
8.3.6.2 Clinics
8.3.6.3 Ambulatory
8.3.6.4 Surgical Centers
8.3.6.5 Home Care
8.3.6.6 Others
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Intravenous Product Packaging Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size by Type
8.4.4.1 IV Bags
8.4.4.2 Cannula
8.4.4.3 Extension Sets
8.4.4.4 Infusion Pumps
8.4.4.5 Stopcocks
8.4.5 Historic and Forecasted Market Size by Material
8.4.5.1 PVC
8.4.5.2 Non-PVC
8.4.5.3 Polyolefin
8.4.5.4 Polypropylene
8.4.6 Historic and Forecasted Market Size by Application
8.4.6.1 Hospitals
8.4.6.2 Clinics
8.4.6.3 Ambulatory
8.4.6.4 Surgical Centers
8.4.6.5 Home Care
8.4.6.6 Others
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Intravenous Product Packaging Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size by Type
8.5.4.1 IV Bags
8.5.4.2 Cannula
8.5.4.3 Extension Sets
8.5.4.4 Infusion Pumps
8.5.4.5 Stopcocks
8.5.5 Historic and Forecasted Market Size by Material
8.5.5.1 PVC
8.5.5.2 Non-PVC
8.5.5.3 Polyolefin
8.5.5.4 Polypropylene
8.5.6 Historic and Forecasted Market Size by Application
8.5.6.1 Hospitals
8.5.6.2 Clinics
8.5.6.3 Ambulatory
8.5.6.4 Surgical Centers
8.5.6.5 Home Care
8.5.6.6 Others
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Intravenous Product Packaging Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size by Type
8.6.4.1 IV Bags
8.6.4.2 Cannula
8.6.4.3 Extension Sets
8.6.4.4 Infusion Pumps
8.6.4.5 Stopcocks
8.6.5 Historic and Forecasted Market Size by Material
8.6.5.1 PVC
8.6.5.2 Non-PVC
8.6.5.3 Polyolefin
8.6.5.4 Polypropylene
8.6.6 Historic and Forecasted Market Size by Application
8.6.6.1 Hospitals
8.6.6.2 Clinics
8.6.6.3 Ambulatory
8.6.6.4 Surgical Centers
8.6.6.5 Home Care
8.6.6.6 Others
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Intravenous Product Packaging Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size by Type
8.7.4.1 IV Bags
8.7.4.2 Cannula
8.7.4.3 Extension Sets
8.7.4.4 Infusion Pumps
8.7.4.5 Stopcocks
8.7.5 Historic and Forecasted Market Size by Material
8.7.5.1 PVC
8.7.5.2 Non-PVC
8.7.5.3 Polyolefin
8.7.5.4 Polypropylene
8.7.6 Historic and Forecasted Market Size by Application
8.7.6.1 Hospitals
8.7.6.2 Clinics
8.7.6.3 Ambulatory
8.7.6.4 Surgical Centers
8.7.6.5 Home Care
8.7.6.6 Others
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Intravenous Product Packaging Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2035 |
|
Historical Data: |
2018 to 2023 |
Market Size in 2024: |
USD 5.69 Bn. |
|
Forecast Period 2025-35 CAGR: |
8.96% |
Market Size in 2035: |
USD 14.62 Bn. |
|
Segments Covered: |
By Type |
|
|
|
By Material |
|
||
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Frequently Asked Questions :
The forecast period in the Intravenous Product Packaging Market research report is 2025–2035.













