Spinal Fusion Devices Market Synopsis:
Spinal Fusion Devices Market Size Was Valued at USD 7.03 Billion in 2023, and is Projected to Reach USD 10.81 Billion by 2032, Growing at a CAGR of 4.90% From 2024-2032.
The spinal fusion devices market is a group of medical tools and technologies used when the vertebrae in the spine are fused to support the healing process required after spinal surgery. These devices include a number of bone grafts, screws, rods, plates and Intervertebral body fusion products used in spine surgery to correct deformities, treat degenerative disc diseases, and spinal trauma. The goals of spinal fusion surgery are focused on elimination of pain and bringing the possibility of mobility improvement by stabilizing the spinal segments where necessary, while the bones heal. It is majorly fueled by the rising cases of spinal disorders as well as improved surgical technology and rising preference of minimally invasive surgeries.
The market for spinal fusion devices is expected to grow due to growth in spinal abnormalities, growth in geriatric population, and new innovations in surgeries. Given that spinal disorders which include herniated discs and spinal stenosis are common in the populace, the need for efficient to treat such disorders has therefore increased. Lumbar spine fusion surgery, which stabilises the spine and relieves pain, is appearing in the literature more frequently as a useful option.
Technology advancement is part of the forces that have put significant influence on the market. Techniques of minimally invasive surgery have stemmed growth in biomaterials and fixation devices improving the patient experience as well as shorter hospital stays. Such improvements have helped in changing the perception on spinal fusion procedures by both the doctors and other persons. In addition, the growing number of outpatient surgeries and higher patient literacy regarding spinal wellness are also believed to spur spinal fusion device sales.
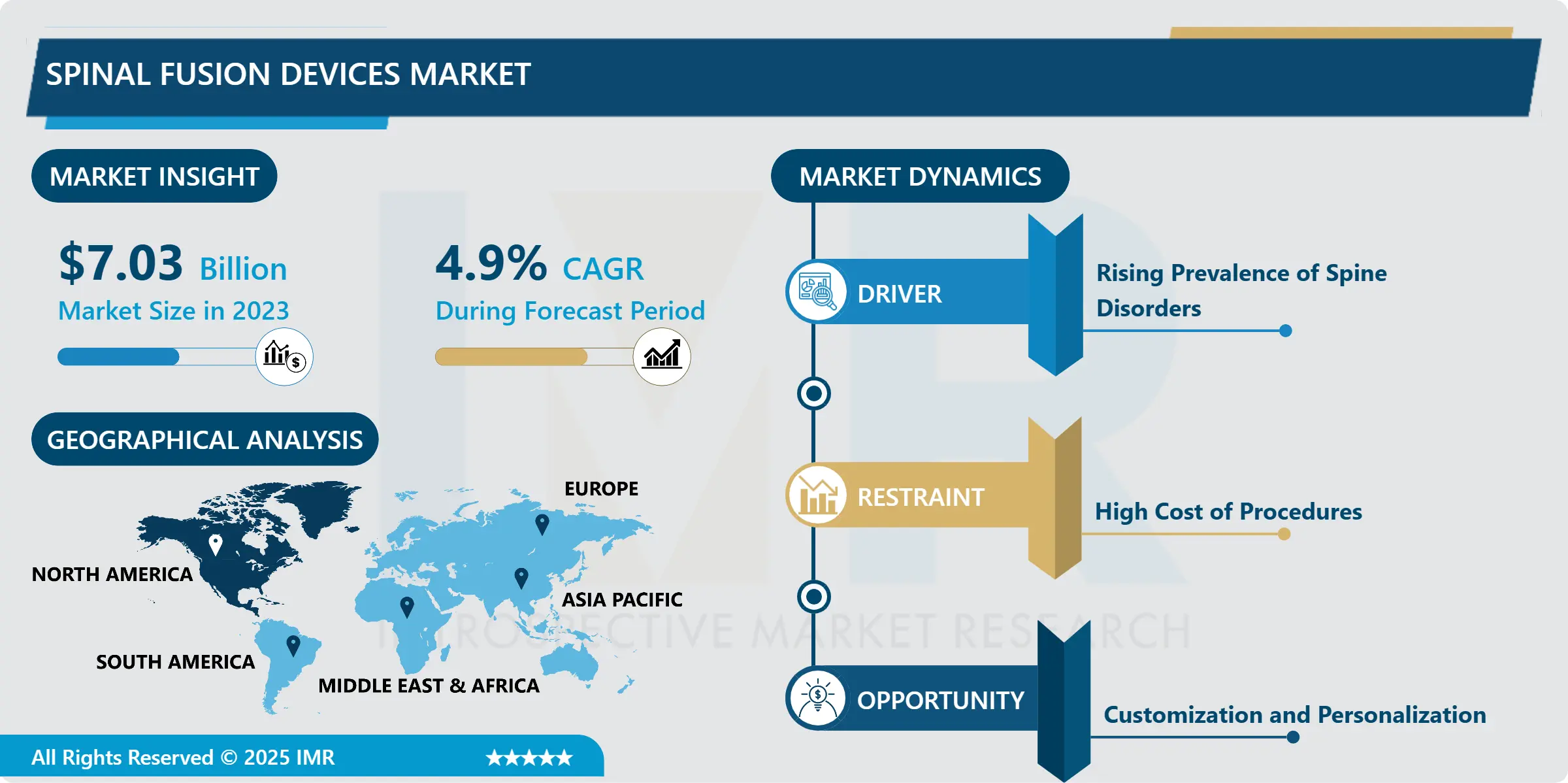
Spinal Fusion Devices Market Trend Analysis:
Market Trends in Spinal Fusion Devices
-
With increasing of world mean age, the incidence of degenerative spinal diseases like herniated discs and spinal stenosis is increasing. These conditions mostly result to chronic pain and use of mobility aids, hence lying barren for surgeries with a view of regaining function and improving lives. The rising prevalence of such illnesses is applying pressure on spinal fusion surgical procedures because hospitals and doctors are in search of good solutions to such illnesses. Demographic changes are also an increasing pool of patients, which provides growth potential to the manufacturers of spinal fusion devices giving them a wider range of products for developing patients.
- Similarly, to the demography trend, the spinal health is now of greater concern to the public. This has boosted the importance of early diagnosis and treatment of back pain or other spinal problems to ensure an individual presents themselves to a physician at an earlier point in their lifetime than their parents’ generation. The prevalence of early detectable spinal conditions labours surgical needs and this includes spinal fusion as a procedure. This is not only driven by improvements in surgeries and instruments but it is also accompanied by patients changing perception towards disease prevention. Consequently, the spinal fusion devices market is set to experience tremendous progress on the basis of demographic changes as well as changing perceptions of healthcare.
Growing Demand for Spinal Fusion Devices Driven by Increasing Prevalence of Disorders and Aging Population
-
New cases of spinal disorders have been posted to register high incidence rates, and this has created a need for operations specifically spinal fusion operations. Diseases like degenerative disc diseases, spinal stenosis, and traumatic spine injuries are emerging more frequent, meaning that there is a huge call for efficient management of the associated symptoms. They are usually characterised by severe pain and restricted movement, and affected individuals will look to undergo surgery in order to improve their quality of life. Conservative treatments like physical therapy and pain management continue to perform poorly, meaning caregivers are advising surgical procedures wherein spinal fusion devices shall record massive growth.
- Also, the increase in the world’s population coupled by the ageing factor has led to increased occurrence of spinal disorders requiring surgery. Besides, people fall ill as they grow older, and it is well known that age may be a critical factor predisposing a person to degenerative arthritis, spasmodic syndromes, and other conditions that may necessitate spinal fusion surgery. Population studies have foreseen that the global population of the geriatric population aged 65 years and above will take a steep rise in the next few decades hence increasing the need for spinal surgeries. Together with gradual increase of the global average age, improvement of surgical methods and development of devices used in spinal fusion devices market are likely to increase the orientation of healthcare facilities on meeting the needs of an aging population.
Spinal Fusion Devices Market Segment Analysis:
Spinal Fusion Devices Market Segmented on the basis of Product, Disease, Surgery, End Use and Region.
By Product, Thoracolumbar Devices segment is expected to dominate the market during the forecast period
-
There is great importance of TL devices in the treatment of different spinal pathology that involves the thoracic and lumbar segments. This group consists of variety of specific kind of products having unique purpose of improving standing or overall spina stability so as to achieve favorable surgical results. Out of those, the anterior lumbar plates play a significant role when spinal fusion is to be performed, as they give required support and alignment to the area to enable proper fusion. They are sited at the anterior aspect of the lumbar spine, and this position makes it easy to distribute the patient’s body weight when regaining balance. Furthermore, such as pedicle screws and rods are also used to fix the vertebrae back into position to restore normal spinal curvature and therefore decrease the pain. They are remarkably helpful in the treatment of diseases including the degenerative disc disease and spinal deformities since they enhance the ability of surgeons to carry out delicate operations.
By End Use, Hospitals segment expected to held the largest share
-
Teaching hospitals represent major centers for intricate spinal surgery, that offers advanced equipment and experienced surgical personnel. Hospitals provide the convenient conditions for thorough preoperative and postoperative investigations and treatments which are considered essential in cases of spinal interventions. Modern techniques like MRI and CT are widely used to diagnose the health conditions and understand the specific biomechanism of injuries before operating on the patient. In addition, working in a hospital setting allows for more integration of multiple professionals into an individual’s care plan thus promoting patient care from a number of different disciplines.
Spinal Fusion Devices Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
-
North American particularly in the U.S the spinal fusion devices market is the largest globally because of high healthcare expenditure and advanced healthcare industry. This is due to the high prevalence of spinal disorders and continued growth of the geriatric population resulting in increased spinal surgeries including those with complex levels that need sophisticated devices. The area is characterized by some of the main players and developers of spinal fusion products and technologies, thus, creating capacity for the development of diverse products to suit a variety of patients. In addition, market with regulatory support and focus on research and development is providing a good support structure for new product launches, which is supporting the overall market growth.
Active Key Players in the Spinal Fusion Devices Market:
-
ATEC Spine, Inc.
- B. Braun SE
- Captiva Spine, Inc.
- Globus Medical
- Institute for Spine & Scoliosis
- Medical Device Business Services, Inc.
- Medtronic
- NuVasive, Inc.
- Orthofix Medical Inc.
- SeaSpine.
- Spine Wave, Inc.
- Stryker
- XTANT MEDICAL
- Zimmer Biomet.
- Other Active Players
|
Global Spinal Fusion Devices Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 7.03 Billion |
|
Forecast Period 2024-32 CAGR: |
4.90% |
Market Size in 2032: |
USD 10.81 Billion |
|
Segments Covered: |
By Product |
|
|
|
By Disease |
|
||
|
By Surgery |
|
||
|
By End Use |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Spinal Fusion Devices Market by Product
4.1 Spinal Fusion Devices Market Snapshot and Growth Engine
4.2 Spinal Fusion Devices Market Overview
4.3 Thoracolumbar Devices
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 Thoracolumbar Devices: Geographic Segmentation Analysis
4.4 Cervical Fixation Devices
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Cervical Fixation Devices: Geographic Segmentation Analysis
4.5 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
4.5.1 Introduction and Market Overview
4.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.5.3 Key Market Trends, Growth Factors and Opportunities
4.5.4 Interbody Fusion Devices) Disease (Other Non-destructive Technologies: Geographic Segmentation Analysis
4.6 Degenerative Disc
4.6.1 Introduction and Market Overview
4.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.6.3 Key Market Trends, Growth Factors and Opportunities
4.6.4 Degenerative Disc: Geographic Segmentation Analysis
4.7 Complex Deformity
4.7.1 Introduction and Market Overview
4.7.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.7.3 Key Market Trends, Growth Factors and Opportunities
4.7.4 Complex Deformity: Geographic Segmentation Analysis
4.8 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
4.8.1 Introduction and Market Overview
4.8.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.8.3 Key Market Trends, Growth Factors and Opportunities
4.8.4 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities) : Geographic Segmentation Analysis
Chapter 5: Company Profiles and Competitive Analysis
5.1 Competitive Landscape
5.1.1 Competitive Benchmarking
5.1.2 Spinal Fusion Devices Market Share by Manufacturer (2023)
5.1.3 Industry BCG Matrix
5.1.4 Heat Map Analysis
5.1.5 Mergers and Acquisitions
5.2 MEDICAL DEVICE BUSINESS SERVICES INC.
5.2.1 Company Overview
5.2.2 Key Executives
5.2.3 Company Snapshot
5.2.4 Role of the Company in the Market
5.2.5 Sustainability and Social Responsibility
5.2.6 Operating Business Segments
5.2.7 Product Portfolio
5.2.8 Business Performance
5.2.9 Key Strategic Moves and Recent Developments
5.2.10 SWOT Analysis
5.3 STRYKER
5.4 ZIMMER BIOMET.
5.5 ORTHOFIX MEDICAL INC.
5.6 B. BRAUN SE
5.7 MEDTRONIC
5.8 NUVASIVE INC.
5.9 GLOBUS MEDICAL
5.10 ATEC SPINE INC
5.11 CAPTIVA SPINE INC.
5.12 SEASPINE
5.13 INSTITUTE FOR SPINE & SCOLIOSIS
5.14 SPINE WAVE INC.
5.15 SPINEOLOGY INC.
5.16 XTANT MEDICAL
5.17 OTHER ACTIVE PLAYERS
Chapter 6: Global Spinal Fusion Devices Market By Region
6.1 Overview
6.2. North America Spinal Fusion Devices Market
6.2.1 Key Market Trends, Growth Factors and Opportunities
6.2.2 Top Key Companies
6.2.3 Historic and Forecasted Market Size by Segments
6.2.4 Historic and Forecasted Market Size By Product
6.2.4.1 Thoracolumbar Devices
6.2.4.2 Cervical Fixation Devices
6.2.4.3 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
6.2.4.4 Degenerative Disc
6.2.4.5 Complex Deformity
6.2.4.6 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
6.2.5 Historic and Forecast Market Size by Country
6.2.5.1 US
6.2.5.2 Canada
6.2.5.3 Mexico
6.3. Eastern Europe Spinal Fusion Devices Market
6.3.1 Key Market Trends, Growth Factors and Opportunities
6.3.2 Top Key Companies
6.3.3 Historic and Forecasted Market Size by Segments
6.3.4 Historic and Forecasted Market Size By Product
6.3.4.1 Thoracolumbar Devices
6.3.4.2 Cervical Fixation Devices
6.3.4.3 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
6.3.4.4 Degenerative Disc
6.3.4.5 Complex Deformity
6.3.4.6 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
6.3.5 Historic and Forecast Market Size by Country
6.3.5.1 Russia
6.3.5.2 Bulgaria
6.3.5.3 The Czech Republic
6.3.5.4 Hungary
6.3.5.5 Poland
6.3.5.6 Romania
6.3.5.7 Rest of Eastern Europe
6.4. Western Europe Spinal Fusion Devices Market
6.4.1 Key Market Trends, Growth Factors and Opportunities
6.4.2 Top Key Companies
6.4.3 Historic and Forecasted Market Size by Segments
6.4.4 Historic and Forecasted Market Size By Product
6.4.4.1 Thoracolumbar Devices
6.4.4.2 Cervical Fixation Devices
6.4.4.3 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
6.4.4.4 Degenerative Disc
6.4.4.5 Complex Deformity
6.4.4.6 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
6.4.5 Historic and Forecast Market Size by Country
6.4.5.1 Germany
6.4.5.2 UK
6.4.5.3 France
6.4.5.4 The Netherlands
6.4.5.5 Italy
6.4.5.6 Spain
6.4.5.7 Rest of Western Europe
6.5. Asia Pacific Spinal Fusion Devices Market
6.5.1 Key Market Trends, Growth Factors and Opportunities
6.5.2 Top Key Companies
6.5.3 Historic and Forecasted Market Size by Segments
6.5.4 Historic and Forecasted Market Size By Product
6.5.4.1 Thoracolumbar Devices
6.5.4.2 Cervical Fixation Devices
6.5.4.3 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
6.5.4.4 Degenerative Disc
6.5.4.5 Complex Deformity
6.5.4.6 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
6.5.5 Historic and Forecast Market Size by Country
6.5.5.1 China
6.5.5.2 India
6.5.5.3 Japan
6.5.5.4 South Korea
6.5.5.5 Malaysia
6.5.5.6 Thailand
6.5.5.7 Vietnam
6.5.5.8 The Philippines
6.5.5.9 Australia
6.5.5.10 New Zealand
6.5.5.11 Rest of APAC
6.6. Middle East & Africa Spinal Fusion Devices Market
6.6.1 Key Market Trends, Growth Factors and Opportunities
6.6.2 Top Key Companies
6.6.3 Historic and Forecasted Market Size by Segments
6.6.4 Historic and Forecasted Market Size By Product
6.6.4.1 Thoracolumbar Devices
6.6.4.2 Cervical Fixation Devices
6.6.4.3 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
6.6.4.4 Degenerative Disc
6.6.4.5 Complex Deformity
6.6.4.6 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
6.6.5 Historic and Forecast Market Size by Country
6.6.5.1 Turkiye
6.6.5.2 Bahrain
6.6.5.3 Kuwait
6.6.5.4 Saudi Arabia
6.6.5.5 Qatar
6.6.5.6 UAE
6.6.5.7 Israel
6.6.5.8 South Africa
6.7. South America Spinal Fusion Devices Market
6.7.1 Key Market Trends, Growth Factors and Opportunities
6.7.2 Top Key Companies
6.7.3 Historic and Forecasted Market Size by Segments
6.7.4 Historic and Forecasted Market Size By Product
6.7.4.1 Thoracolumbar Devices
6.7.4.2 Cervical Fixation Devices
6.7.4.3 Interbody Fusion Devices) Disease (Other Non-destructive Technologies
6.7.4.4 Degenerative Disc
6.7.4.5 Complex Deformity
6.7.4.6 Trauma & Fractures and Others) Surgery (Open Spine Surgery and Minimally Invasive Spine Surgery) End Use (Hospitals and Outpatient Facilities)
6.7.5 Historic and Forecast Market Size by Country
6.7.5.1 Brazil
6.7.5.2 Argentina
6.7.5.3 Rest of SA
Chapter 7 Analyst Viewpoint and Conclusion
7.1 Recommendations and Concluding Analysis
7.2 Potential Market Strategies
Chapter 8 Research Methodology
8.1 Research Process
8.2 Primary Research
8.3 Secondary Research
|
Global Spinal Fusion Devices Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 7.03 Billion |
|
Forecast Period 2024-32 CAGR: |
4.90% |
Market Size in 2032: |
USD 10.81 Billion |
|
Segments Covered: |
By Product |
|
|
|
By Disease |
|
||
|
By Surgery |
|
||
|
By End Use |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













