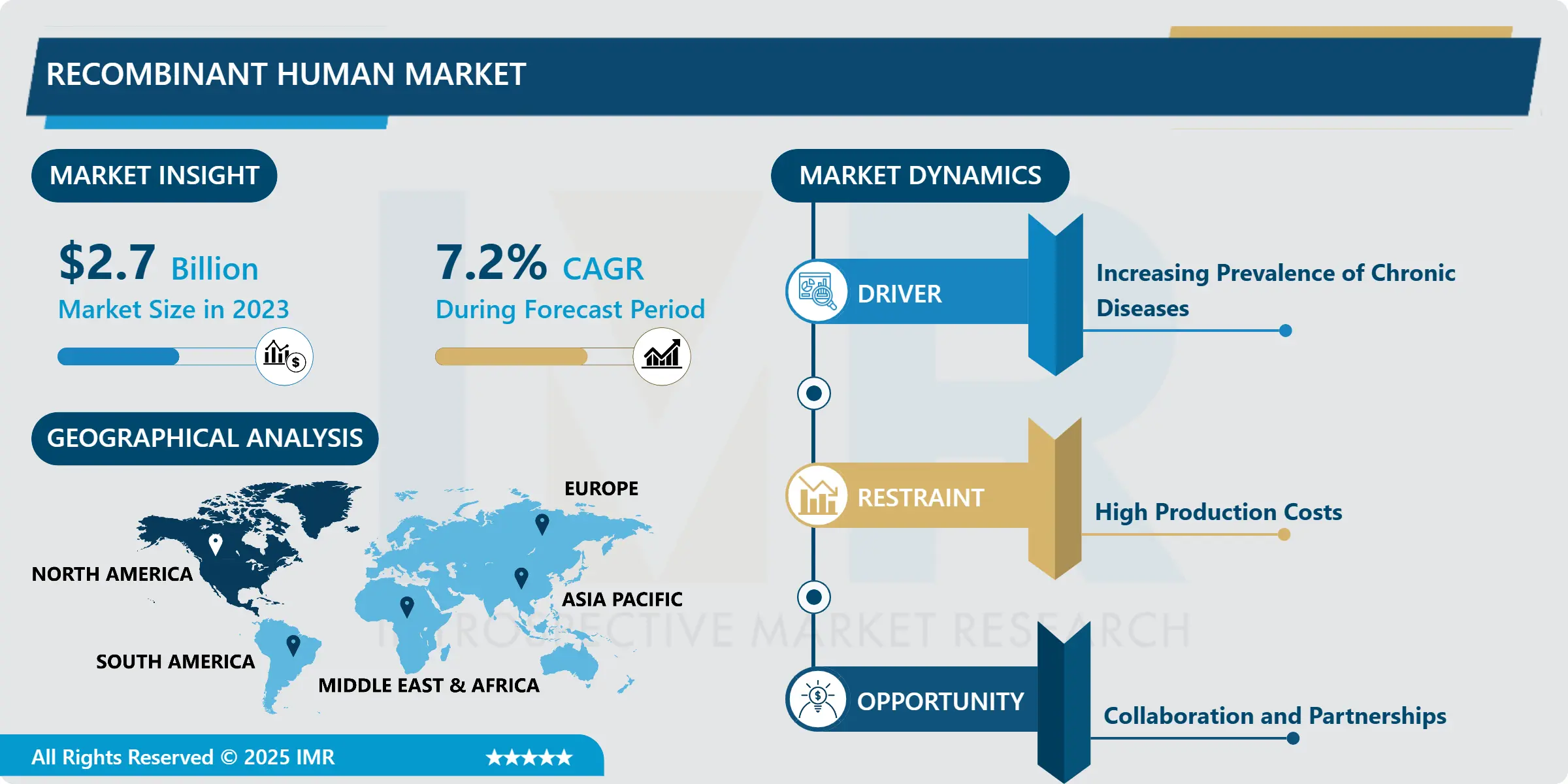
Recombinant Human Market Synopsis:
Recombinant Human Market Size Was Valued at USD 2.7 Billion in 2023 and is Projected to Reach USD 5.05 Billion by 2032, Growing at a CAGR of 7.2% From 2024-2032.
The recombinant human market is a sub-sector in the biotechnology industry dealing in the generation and marketing of recombinant human proteins which are formulated from recombinant DNA technology. These proteins are incorporated in a numerous number therapeutic approaches, for chronic diseases, inherited diseases, and diseases such as diabetes, haemophilia, and cancers. The market comprises a very broad array of products such as hormones, monoclonal antibodies, blood factors, enzymes etc. which are now being developed and employed more often because of various advantages in terms of efficacy and safety as well as producibility as opposed to traditional methods of production. This market is growing due to the developments in biomanufacturing technique, increased funding on biotech research, and elevated incidence of diseases involving protein therapy.
There is currently huge growth in the recombinant human market due to the new technology that is being developed in the biotechnology, and the ever growing number of people suffering from chronic diseases which warrants the need of more effective therapeutic products.. Human recombinant proteins genus made by using recombinant DNA technology are very significant for curing different diseases, a lot of them are genetic diseases and cancers, metabolic diseases. The specificity of the market can be described as diverse featuring such products as hormones, enzymes, and monoclonal antibodies unique solutions changing the market. Major market stakeholders have currently increased their research nous on product performance and safety, which has led to emergence of new drugs.
There is currently huge growth in the recombinant human market due to the new technology that is being developed in the biotechnology, and the ever growing number of people suffering from chronic diseases which warrants the need of more effective therapeutic products. Human recombinant proteins genus made by using recombinant DNA technology are very significant for curing different diseases, a lot of them are genetic diseases and cancers, metabolic diseases. The specificity of the market can be described as diverse featuring such products as hormones, enzymes, and monoclonal antibodies unique solutions changing the market. Major market stakeholders have currently increased their research nous on product performance and safety, which has led to emergence of new drugs.
Recombinant Human Market Trend Analysis
Trend
Advancements and Growth Drivers
- Organised sector for recombinant human is undergoing transition to a new era that has seen a shift influenced highly by developments in genetic engineering.. This progress has supported the production of a complete spectrum of recombinant products used in treatment of various diseases like diabetes, hemophilia and growth disorders. The number of recombinant therapeutic proteins products including insulin, erythropoietin, and monoclonal antibodies are increasing, demonstrating their importance in chronic disease treatment. These therapies not only improve the quality of life for patients but are also an important part of treatment in today’s treatment needs for individuals with chronic health care conditions.
- In addition, the research reviews of high authorities such as FDA and Health Canada state that medical facilities are required to use high quality, sterile respiratory disposables. These regulations combined with considerable focus on the clinical settings amid the infection control create a favorable environment for the market growth. The continuous drive to embracing best practices measure – primarily sparked on by the COVID-19 pandemic – has addressed concerns on cross-contamination thus increasing the use of single-use respiratory devices. Responsiveness to these regulations, development of advanced healthcare market and increased number of patients make North America a large and competitive market for respiratory disposables.
Opportunity
The Shift Toward Personalized Medicine
- The evolution towards molecular medicine has greatly amplified the demand in manufacture of human proteins such as recombinant ones for matching the patient demands. These proteins play crucial roles in gene and stem cell therapies – both of which are important areas for advancement in the present and near future. Oncological pathology, congenital abnormalities, and some metabolic disorders are known to be treatable by agents which take advantage of the unique qualities of recombinant proteins, thus improving therapeutic outcomes. In the course of disease management, there is greater emphasis on the use of products developed for individual patients, and the use of these recombinant human proteins will grow correspondingly, reinforcing their importance in today’s medicine.
- There is a continuous practice of R&D in biotechnology that provides an opportunity for new therapeutic applications of recombinant human proteins.. The development of the new genetic engineering biomanufacturing has enabled the researchers to develop improved proteins and widen the treatable disease spectrums. Moreover, the cooperation between universities and the pharmaceutical industry is increasing and efficiently promotes the commercialization of research. While these efforts remain in place, it is envisaged that they will fuel comes the growth of the recombinant human market, prompting development of more products that can effectively address the newer need that patients and care givers present in the market.
Recombinant Human Market Segment Analysis:
Recombinant Human Market Segmented on the basis of By Product Type, By Application and By End User
By Product Type, Recombinant Proteins segment is expected to dominate the market during the forecast period
- There is a continuous practice of R&D in biotechnology that provides an opportunity for new therapeutic applications of recombinant human proteins.. The development of the new genetic engineering biomanufacturing has enabled the researchers to develop improved proteins and widen the treatable disease spectrums. Moreover, the cooperation between universities and the pharmaceutical industry is increasing and efficiently promotes the commercialization of research. While these efforts remain in place, it is envisaged that they will fuel comes the growth of the recombinant human market, prompting development of more products that can effectively address the newer need that patients and care givers present in the market.
- There is a continuous practice of R&D in biotechnology that provides an opportunity for new therapeutic applications of recombinant human proteins.. The development of the new genetic engineering biomanufacturing has enabled the researchers to develop improved proteins and widen the treatable disease spectrums. Moreover, the cooperation between universities and the pharmaceutical industry is increasing and efficiently promotes the commercialization of research. While these efforts remain in place, it is envisaged that they will fuel comes the growth of the recombinant human market, prompting development of more products that can effectively address the newer need that patients and care givers present in the market.
By End User, Pharmaceutical Companies segment expected to held the largest share
- Players of biologic therapies also include the pharmaceutical industry is in charge of the research, production, and marketing of biologic products.. These organizations are usually vested with strong research undertakings mostly with funds committed to Research and Development to develop new treatment forms or vaccines. This grim commitment to meet the unmet medical needs with biologics is in tandem with the broader movement towards efficient treatment platforms within the industry. With the current change in the delivery of health services, drug makers are shifting their attention to personalized medicine and precision health as they strive to reach out for an optimization of the prescribing of drugs through a better analysis on the resultant effects to the patient’s profile.
- Players of biologic therapies also include the pharmaceutical industry is in charge of the research, production, and marketing of biologic products.. These organizations are usually vested with strong research undertakings mostly with funds committed to Research and Development to develop new treatment forms or vaccines. This grim commitment to meet the unmet medical needs with biologics is in tandem with the broader movement towards efficient treatment platforms within the industry. With the current change in the delivery of health services, drug makers are shifting their attention to personalized medicine and precision health as they strive to reach out for an optimization of the prescribing of drugs through a better analysis on the resultant effects to the patient’s profile.
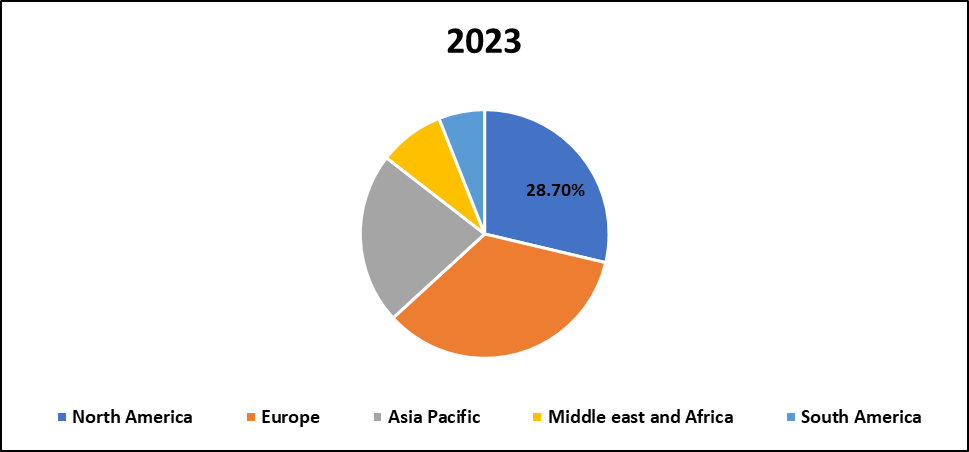
Recombinant Human Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- The North American recombinant human market is more developed in terms of healthcare industry and a large amount of investment in biotechnology research and development. There are many renowned pharmaceutical manufacturers in the said area that have continued to bring about development of therapeutic proteins. Strong accessory regulatory systems test that recombinant products have suitable safety and efficacy, and increase the level of confidence in consumers as well as stimulate the growth of the market. The high priority of the personalized choice in North America means that the treatment is carefully chosen according to the patient’s requirements, which is particularly suitable for the growth of the precision medicine that gradually gains more popularity all over the world.
- Especially in the United States, massive sponsorship of biomedical research is central to the continued market drive.. Adoption of the recombinant technology is packaged with a already developed distribution network within the nation. Moreover, because humans are now experiencing chronic diseases and genetic disorders more frequently, there is a pressure to develop more effective treatments for recombinant products. University-industry partnerships combined with expansion of mergers and acquisitions will put further dynamism to the competitive structure and advance innovative solutions and better patient experience in the North American recombinant human market.
Recombinant Human Market Share, by Geography, 2023 (%)
Active Key Players in the Recombinant Human Market
- Amgen Inc.
- Genentech, a member of the Roche Group
- Novo Nordisk
- Sanofi
- Bristol-Myers Squibb
- Johnson & Johnson
- Merck & Co., Inc.
- Pfizer Inc.
- AbbVie Inc.
- Regeneron Pharmaceuticals, Inc.
- Other key Players
|
Recombinant Human Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 2.7 Billion |
|
Forecast Period 2024-32 CAGR: |
7.2% |
Market Size in 2032: |
USD 5.05 Billion |
|
Segments Covered: |
By Product Type |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Recombinant Human Market by Product Type
4.1 Recombinant Human Market Snapshot and Growth Engine
4.2 Recombinant Human Market Overview
4.3 Recombinant Proteins
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 Recombinant Proteins: Geographic Segmentation Analysis
4.4 Recombinant Vaccines
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Recombinant Vaccines: Geographic Segmentation Analysis
4.5 Gene Therapies and Cellular Therapies
4.5.1 Introduction and Market Overview
4.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.5.3 Key Market Trends, Growth Factors and Opportunities
4.5.4 Gene Therapies and Cellular Therapies: Geographic Segmentation Analysis
Chapter 5: Recombinant Human Market by Application
5.1 Recombinant Human Market Snapshot and Growth Engine
5.2 Recombinant Human Market Overview
5.3 Therapeutic Applications
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Therapeutic Applications: Geographic Segmentation Analysis
5.4 Preventive Applications and Diagnostic Applications
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Preventive Applications and Diagnostic Applications: Geographic Segmentation Analysis
Chapter 6: Recombinant Human Market by End User
6.1 Recombinant Human Market Snapshot and Growth Engine
6.2 Recombinant Human Market Overview
6.3 Pharmaceutical Companies
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Pharmaceutical Companies: Geographic Segmentation Analysis
6.4 Research Institutions
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 Research Institutions: Geographic Segmentation Analysis
6.5 Biotechnology Companies and Hospitals and Clinics
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 Biotechnology Companies and Hospitals and Clinics: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Recombinant Human Market Share by Manufacturer (2023)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 AMGEN INC.
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 GENENTECH
7.4 A MEMBER OF THE ROCHE GROUP
7.5 NOVO NORDISK
7.6 SANOFI
7.7 BRISTOL-MYERS SQUIBB
7.8 JOHNSON & JOHNSON
7.9 MERCK & CO. INC.
7.10 PFIZER INC.
7.11 ABBVIE INC.
7.12 REGENERON PHARMACEUTICALS INC.
7.13 OTHER ACTIVE PLAYERS
Chapter 8: Global Recombinant Human Market By Region
8.1 Overview
8.2. North America Recombinant Human Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By Product Type
8.2.4.1 Recombinant Proteins
8.2.4.2 Recombinant Vaccines
8.2.4.3 Gene Therapies and Cellular Therapies
8.2.5 Historic and Forecasted Market Size By Application
8.2.5.1 Therapeutic Applications
8.2.5.2 Preventive Applications and Diagnostic Applications
8.2.6 Historic and Forecasted Market Size By End User
8.2.6.1 Pharmaceutical Companies
8.2.6.2 Research Institutions
8.2.6.3 Biotechnology Companies and Hospitals and Clinics
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Recombinant Human Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By Product Type
8.3.4.1 Recombinant Proteins
8.3.4.2 Recombinant Vaccines
8.3.4.3 Gene Therapies and Cellular Therapies
8.3.5 Historic and Forecasted Market Size By Application
8.3.5.1 Therapeutic Applications
8.3.5.2 Preventive Applications and Diagnostic Applications
8.3.6 Historic and Forecasted Market Size By End User
8.3.6.1 Pharmaceutical Companies
8.3.6.2 Research Institutions
8.3.6.3 Biotechnology Companies and Hospitals and Clinics
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Recombinant Human Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By Product Type
8.4.4.1 Recombinant Proteins
8.4.4.2 Recombinant Vaccines
8.4.4.3 Gene Therapies and Cellular Therapies
8.4.5 Historic and Forecasted Market Size By Application
8.4.5.1 Therapeutic Applications
8.4.5.2 Preventive Applications and Diagnostic Applications
8.4.6 Historic and Forecasted Market Size By End User
8.4.6.1 Pharmaceutical Companies
8.4.6.2 Research Institutions
8.4.6.3 Biotechnology Companies and Hospitals and Clinics
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Recombinant Human Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By Product Type
8.5.4.1 Recombinant Proteins
8.5.4.2 Recombinant Vaccines
8.5.4.3 Gene Therapies and Cellular Therapies
8.5.5 Historic and Forecasted Market Size By Application
8.5.5.1 Therapeutic Applications
8.5.5.2 Preventive Applications and Diagnostic Applications
8.5.6 Historic and Forecasted Market Size By End User
8.5.6.1 Pharmaceutical Companies
8.5.6.2 Research Institutions
8.5.6.3 Biotechnology Companies and Hospitals and Clinics
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Recombinant Human Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By Product Type
8.6.4.1 Recombinant Proteins
8.6.4.2 Recombinant Vaccines
8.6.4.3 Gene Therapies and Cellular Therapies
8.6.5 Historic and Forecasted Market Size By Application
8.6.5.1 Therapeutic Applications
8.6.5.2 Preventive Applications and Diagnostic Applications
8.6.6 Historic and Forecasted Market Size By End User
8.6.6.1 Pharmaceutical Companies
8.6.6.2 Research Institutions
8.6.6.3 Biotechnology Companies and Hospitals and Clinics
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Recombinant Human Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By Product Type
8.7.4.1 Recombinant Proteins
8.7.4.2 Recombinant Vaccines
8.7.4.3 Gene Therapies and Cellular Therapies
8.7.5 Historic and Forecasted Market Size By Application
8.7.5.1 Therapeutic Applications
8.7.5.2 Preventive Applications and Diagnostic Applications
8.7.6 Historic and Forecasted Market Size By End User
8.7.6.1 Pharmaceutical Companies
8.7.6.2 Research Institutions
8.7.6.3 Biotechnology Companies and Hospitals and Clinics
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Recombinant Human Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 2.7 Billion |
|
Forecast Period 2024-32 CAGR: |
7.2% |
Market Size in 2032: |
USD 5.05 Billion |
|
Segments Covered: |
By Product Type |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













