Materiovigilance Market Synopsis:
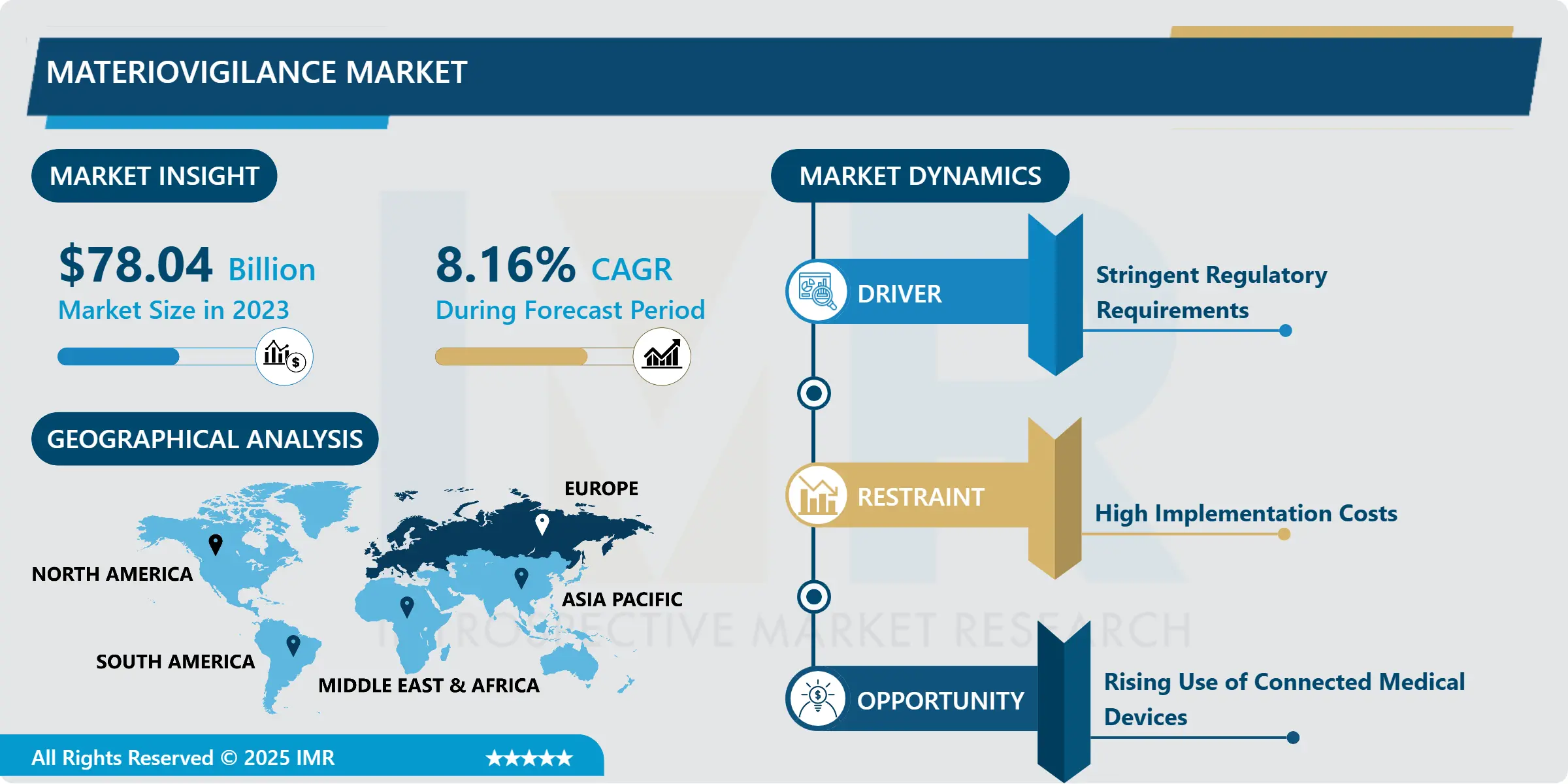
Materiovigilance Market Size Was Valued at USD 78.04 Billion in 2023, and is Projected to Reach USD 158.09 Billion by 2032, Growing at a CAGR of 8.16% From 2024-2032.
Materiovigilance is the act of tracking and reviewing the safety profile of medical devices with a specific aim of identifying, comparing and addressing all possible adverse outcomes commonly known as incidents associated with the use of medical devices. The purpose is to address risks to patients’ safety through: o Identifying potential lifesaving and/or effective medical devices risks Verifying compliance with recognised safety and performance standards at each stage of a device’s lifecycle.
The Materiovigilance market has grown popular because of the growing demand of medical devices in all kinds of healthcare facilities, always ranging from diagnostic to therapeutic to surgical devices. With the health institutions all embracing increased use of sophisticated medical technologies in the delivery of healthcare it has become paramount to guard the safety of these gadgets. Materiovigilance systems monitor adverse events, device failures or any situation that could be potentially dangerous for the patient, supplying a wide-ranging functional model to operate after the market launch of medical devices.
This strategic roadmap remains under the control of governments and regulatory authorities with stringent guidelines in the European region under the European Medical Device Regulation (MDR) and the United States under the Medical Device Reporting Regulations. Continuing with this trend, digital health technologies are accelerating the materiovigilance market due to greater integration of devices and software solutions into healthcare systems. As a result, there is increasing demand in monitoring systems that can check device-related injuries within the healthcare industry across the globe this has fostered the market growth of materiovigilance solutions.
Materiovigilance Market Trend Analysis:
Digital Transformation in Materiovigilance
-
The Materiovigilance conscious market has seen a rise in the digital transformation of surveillance processes as a trend. Hence, with increasing usage of AI, ML, and big data, organizations have devised the ways to utilize these technologies for odd event reporting and risk evaluation. Current artificial solutions can effectively look through the significant amount of data collected from different sources ranging from patients’ records to clinical trials in search of safety signals.
- The use of Cloud based solutions is another aspect of Digital transformation in this sector. The above platforms bring issues of scalability, possibility to have real time information and remote monitoring of events hence improving the relation between manufacturers, health facilities and the regulatory body. With the increased use of digital health and connectivity, there will be an even more significant need for digital materiovigilance systems needed to detect and monitor hazards relating to personal medical devices.
Rising Use of Connected Medical Devices
-
The growing levels of connectivity in medical devices also point to another major opportunity to expand the materiovigilance market. These include, wearables, remote monitoring systems, and implantable devices that produce massive real-time patient data to monitor performance and safety. This volume of information can be utilized to identify trends signaling when something is wrong or likely to go wrong, and stop it before it causes damage to patients.
- As the concept of the Internet of Medical Things continues to develop and grow, there will be a high need for better materiovigilance systems to deal with the increased amounts of data. Thus, the companies that are able to design solutions, which are compatible with IoMT systems and leased for real-time control over the safety conditions of devices, will benefit from this opportunity most of all. Moreover, the regulatory authority is expected to further tighten the reins on smart and connected devices, which will only add pressure to the implementation of cost-effective materiovigilance solutions.
Materiovigilance Market Segment Analysis:
Materiovigilance Market is segmented on the basis of Delivery Mode, Application, End User, and Region.
By Delivery Mode, Cloud-Based segment is expected to dominate the market during the forecast period
-
The materiovigilance market offers two primary delivery modes for surveillance systems: on premise and cloud. On-premise solutions refer to the implementation of the materiovigilance software using the organization’s physical structures, it offers the organizations full control on data and system security. These solutions are especially popular in organizations which aim at protecting personal data of their users and avoiding legal issues, particularly in the countries with strict legislation in data protection.
- While wide and deep solutions are implemented based on hybrid models that incorporate offsite solutions, there are increasing trends in cloud-based solutions for the following reasons: flexibility, scalability and cost factors. Cloud platforms provide an ability to share real-time data across many sites depending on manufacturers’ locations so that the adverse events can be easily monitored and compliance with the requirements of the regulatory authorities can be met. Demand for remote and automated systems is growing, therefore materiovigilance platforms based on the cloud will also show active development.
By Application, Therapeutic segment expected to held the largest share
-
Materiovigilance systems are common in all classes of medical devices that are used in diagnosing, treating, operating on the patient, and utilizing in research. Special instruments include imaging systems as well as diagnostic kits that have to be monitored constantly thanks to the need of receiving accurate results and ensuring that the process is safe for the patient. Any failure or error displayed by these devices may result in wrong diagnoses and dangerous health consequences; hence, materiovigilance is important for this application.
- Other medical products that cannot be removed from the list of potentially dangerous products include therapeutic and surgical devices including implantable devices and robotic surgical systems which have a direct impact on patient health during the treatment. When it comes to research applications, materiovigilance has a very important role when it comes to testing the safety of experimental devices and prototypes destined for clinical use. While the industry keeps on expanding its innovative products and services in the mentioned segments, the materiovigilance requirements for each application will also increase.
Materiovigilance Market Regional Insights:
Europe is Expected to Dominate the Market Over the Forecast period
-
The materiovigilance market is concentrated in Europe due to the enforcement of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR). These regulations have greatly enhanced post-market obligation standards for sought medical devices, and they force manufacturers to spend funds on materiovigilance systems so as to conform to these requirements. The MDR specifically requires increased reporting of adverse events, making there is higher need for effective surveillance systems.
- This is coupled with the Europe having a highly advanced healthcare structure, Technology advancement, and large number of medical device manufacturers serving this market. Due to ongoing changes in the legal aspects in Europe, the materiovigilance market is likely to grow even more as an increasing level of enterprises starts applying sophisticated monitoring systems to fulfill the necessary regulation.
Active Key Players in the Materiovigilance Market:
-
Abbott Laboratories (USA)
- Medtronic (Ireland)
- GE Healthcare (USA)
- Philips Healthcare (Netherlands)
- Siemens Healthineers (Germany)
- Johnson & Johnson (USA)
- Boston Scientific (USA)
- B. Braun Melsungen AG (Germany)
- Stryker Corporation (USA)
- Zimmer Biomet (USA)
- Roche Diagnostics (Switzerland)
- Smith & Nephew (UK)
- Other Active Players
|
Materiovigilance Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 78.04 Billion |
|
Forecast Period 2024-32 CAGR: |
8.16% |
Market Size in 2032: |
USD 158.09 Billion |
|
Segments Covered: |
By Delivery Mode |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Materiovigilance Market by Delivery Mode
4.1 Materiovigilance Market Snapshot and Growth Engine
4.2 Materiovigilance Market Overview
4.3 On-premise
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 On-premise: Geographic Segmentation Analysis
4.4 Cloud-Based
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Cloud-Based: Geographic Segmentation Analysis
Chapter 5: Materiovigilance Market by Application
5.1 Materiovigilance Market Snapshot and Growth Engine
5.2 Materiovigilance Market Overview
5.3 Diagnostic Application
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Diagnostic Application: Geographic Segmentation Analysis
5.4 Therapeutic Application
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Therapeutic Application: Geographic Segmentation Analysis
5.5 Surgical Application
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 Surgical Application: Geographic Segmentation Analysis
5.6 Research Application
5.6.1 Introduction and Market Overview
5.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.6.3 Key Market Trends, Growth Factors and Opportunities
5.6.4 Research Application: Geographic Segmentation Analysis
5.7 Others
5.7.1 Introduction and Market Overview
5.7.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.7.3 Key Market Trends, Growth Factors and Opportunities
5.7.4 Others: Geographic Segmentation Analysis
Chapter 6: Materiovigilance Market by End Users
6.1 Materiovigilance Market Snapshot and Growth Engine
6.2 Materiovigilance Market Overview
6.3 Contract Research Organization
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Contract Research Organization: Geographic Segmentation Analysis
6.4 Business Process Outsourcing
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 Business Process Outsourcing: Geographic Segmentation Analysis
6.5 Original Equipment Manufacturers
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 Original Equipment Manufacturers: Geographic Segmentation Analysis
6.6 Others
6.6.1 Introduction and Market Overview
6.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.6.3 Key Market Trends, Growth Factors and Opportunities
6.6.4 Others: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Materiovigilance Market Share by Manufacturer (2023)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 ABBOTT LABORATORIES (USA)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 MEDTRONIC (IRELAND)
7.4 GE HEALTHCARE (USA)
7.5 PHILIPS HEALTHCARE (NETHERLANDS)
7.6 SIEMENS HEALTHINEERS (GERMANY)
7.7 JOHNSON & JOHNSON (USA)
7.8 BOSTON SCIENTIFIC (USA)
7.9 B. BRAUN MELSUNGEN AG (GERMANY)
7.10 STRYKER CORPORATION (USA)
7.11 OTHER ACTIVE PLAYERS
Chapter 8: Global Materiovigilance Market By Region
8.1 Overview
8.2. North America Materiovigilance Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By Delivery Mode
8.2.4.1 On-premise
8.2.4.2 Cloud-Based
8.2.5 Historic and Forecasted Market Size By Application
8.2.5.1 Diagnostic Application
8.2.5.2 Therapeutic Application
8.2.5.3 Surgical Application
8.2.5.4 Research Application
8.2.5.5 Others
8.2.6 Historic and Forecasted Market Size By End Users
8.2.6.1 Contract Research Organization
8.2.6.2 Business Process Outsourcing
8.2.6.3 Original Equipment Manufacturers
8.2.6.4 Others
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Materiovigilance Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By Delivery Mode
8.3.4.1 On-premise
8.3.4.2 Cloud-Based
8.3.5 Historic and Forecasted Market Size By Application
8.3.5.1 Diagnostic Application
8.3.5.2 Therapeutic Application
8.3.5.3 Surgical Application
8.3.5.4 Research Application
8.3.5.5 Others
8.3.6 Historic and Forecasted Market Size By End Users
8.3.6.1 Contract Research Organization
8.3.6.2 Business Process Outsourcing
8.3.6.3 Original Equipment Manufacturers
8.3.6.4 Others
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Materiovigilance Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By Delivery Mode
8.4.4.1 On-premise
8.4.4.2 Cloud-Based
8.4.5 Historic and Forecasted Market Size By Application
8.4.5.1 Diagnostic Application
8.4.5.2 Therapeutic Application
8.4.5.3 Surgical Application
8.4.5.4 Research Application
8.4.5.5 Others
8.4.6 Historic and Forecasted Market Size By End Users
8.4.6.1 Contract Research Organization
8.4.6.2 Business Process Outsourcing
8.4.6.3 Original Equipment Manufacturers
8.4.6.4 Others
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Materiovigilance Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By Delivery Mode
8.5.4.1 On-premise
8.5.4.2 Cloud-Based
8.5.5 Historic and Forecasted Market Size By Application
8.5.5.1 Diagnostic Application
8.5.5.2 Therapeutic Application
8.5.5.3 Surgical Application
8.5.5.4 Research Application
8.5.5.5 Others
8.5.6 Historic and Forecasted Market Size By End Users
8.5.6.1 Contract Research Organization
8.5.6.2 Business Process Outsourcing
8.5.6.3 Original Equipment Manufacturers
8.5.6.4 Others
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Materiovigilance Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By Delivery Mode
8.6.4.1 On-premise
8.6.4.2 Cloud-Based
8.6.5 Historic and Forecasted Market Size By Application
8.6.5.1 Diagnostic Application
8.6.5.2 Therapeutic Application
8.6.5.3 Surgical Application
8.6.5.4 Research Application
8.6.5.5 Others
8.6.6 Historic and Forecasted Market Size By End Users
8.6.6.1 Contract Research Organization
8.6.6.2 Business Process Outsourcing
8.6.6.3 Original Equipment Manufacturers
8.6.6.4 Others
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Materiovigilance Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By Delivery Mode
8.7.4.1 On-premise
8.7.4.2 Cloud-Based
8.7.5 Historic and Forecasted Market Size By Application
8.7.5.1 Diagnostic Application
8.7.5.2 Therapeutic Application
8.7.5.3 Surgical Application
8.7.5.4 Research Application
8.7.5.5 Others
8.7.6 Historic and Forecasted Market Size By End Users
8.7.6.1 Contract Research Organization
8.7.6.2 Business Process Outsourcing
8.7.6.3 Original Equipment Manufacturers
8.7.6.4 Others
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Materiovigilance Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 78.04 Billion |
|
Forecast Period 2024-32 CAGR: |
8.16% |
Market Size in 2032: |
USD 158.09 Billion |
|
Segments Covered: |
By Delivery Mode |
|
|
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













