Global MEMS For Therapeutic Market Overview
MEMS for Therapeutic Market Size Was Valued at USD 9.39 Billion In 2023 And Is Projected to Reach USD 27.54 Billion By 2032, Growing at A CAGR of 12.7% From 2024-2032.
The origins of MEMS technologies can be traced back to 1954 when Smith published a paper entitled ""Piezoresistive effects in silicon and germanium"" in the international journal ""Physical Review"". Since then, advancements in technologies opened doors for the development of MEMS for healthcare needs. In medicine, therapeutics is related to the care of the patient and includes preventing as well as managing disease or disease-specific problems. Microtechnology and MEMS (microelectromechanical systems) have transformed and in the upcoming future will continue to revolutionize therapeutic medicine as more technological advances are made. Many companies have launched micro-sized devices that have sensors and are utilized to monitor pressure, temperature, and oxygenation level. Several companies have started working on new devices to change the therapeutics procedure. A narrow therapeutic window is the main challenge to the conventional drug release system and this can be overcome by employing MEMS therapeutic solutions. For instance, several studies are being carried out to check the potential of the MEMS sensors in continuous glucose monitoring systems. The growing awareness about the benefits of MEMS in therapeutics is propelling the expansion of MEMS for the therapeutics market over the forecast period.
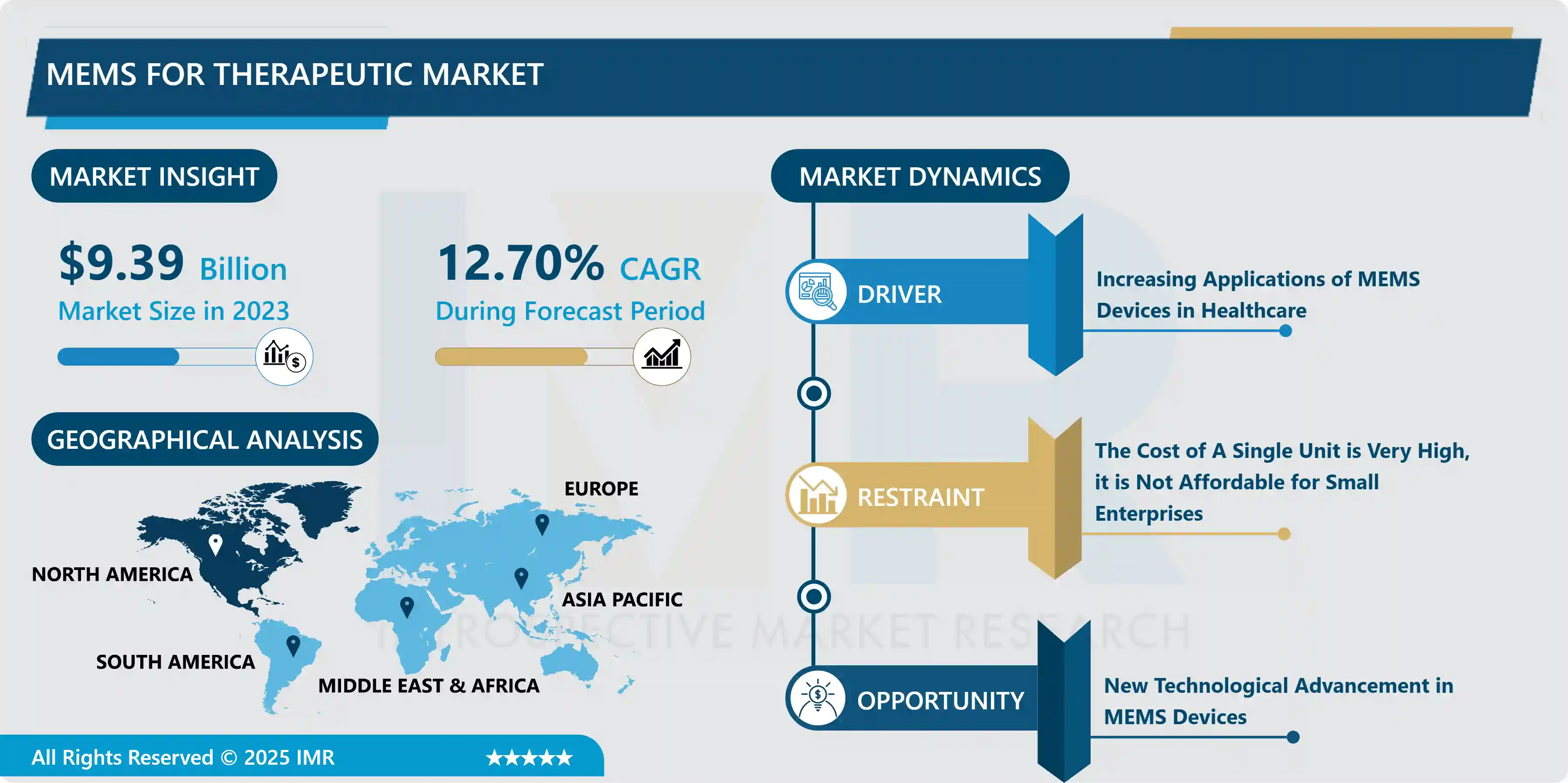
Market Dynamics And Key Factors of MEMS For Therapeutic Market
Drivers:
- Increasing applications of MEMS devices in healthcare and the growth in the personal healthcare market including wireless implants are driving the expansion of the market during the forecast period. The rise in the awareness and affordability of healthcare services has further propelled the demand for MEMS devices in the medical industry. Early detection and treatment of disease are paramount for improving human health and wellness. MEMS devices enable new opportunities for the quick, cost-effective, and precise detection of abnormal biological states that can detect the early onset of disease. MEMS devices can function at a scale more sensitive to numerous biological processes.
- MEMS for therapeutics are mostly sensors that can be employed inside or on the human body to monitor the functioning of vital organs. Implantable biomedical MEMS devices are incorporated to detect the presence of disease-specific biomarkers. On the body, devices gather and process information. Combination devices detect, monitor, and manage the disease. Bio-sensing is a vast concept that includes disease diagnosis to provide physicians with valuable data, regarding blood oxygen levels, and intraocular or aortic pressure. MEMS for therapeutic sensors are lab on chip concept. Moreover, MEMS medical sensors have given rise to microfluidics-it is a study of the behavior of fluids through micro-channels, and the technology of manufacturing microminiaturized devices to detect the presence of harmful microorganisms.
- The rise in the number of individuals suffering from diabetes, hypertension, depression, and other chronic illness has boosted the growth of the MEMS for the therapeutic market. Usage of MEMS for monitoring services has given rise to a continuous monitoring system such as glucose monitoring systems. For instance, an implanted glucose-sensing element detects an increase in glucose levels, this information is sent to an external insulin pump which then injects insulin to counteract the increased glucose levels. Wireless implantable continuous glucose monitoring system employs an array of platinum electrodes with corresponding silver/silver chloride reference electrodes to monitor the two-step enzymatic reaction of glucose oxidase and catalase which is compared to an oxygen-sensing reference.
Restraints:
- High costs involved during the research and development of new MEMS devices for therapeutic usage, and the expensive upfront cost required for the fabrication of cleanrooms and foundry facilities are the vital factors that will hamper the growth of the MEMS for the therapeutic market over the forecast period. Moreover, the cost of a single unit is very high and if they are utilized for niche applications, the desired outcome can't be achieved. Developing countries lack the expertise and superior infrastructure to support the growth of the MEMS for the therapeutic market. Government regulations and the non-acceptance of new technologies in diagnostics pose restraining factors for MEMS for the therapeutic market.
Opportunities:
- The constant demand for improved efficacy, safety, and functionality in in-vivo therapeutics opens up endless opportunities for players involved in MEMS for the therapeutic market. The rise in the number of research activities to improve the functionality of MEMS devices by enhancing sensitivity, specificity, and the integration of these devices with biological processes will bolster medical science with novel MEMS-based diagnostics and therapeutics. New technological advancement in MEMS devices enables the creation and integration of MEMS devices into Microfluidic systems and cell sorting devices. Separation methods offered by microfluidic system technology can potentially manipulate individual cells, reduce reagent costs, enable high-volume experiments, and separate and isolate areas of micro structuring of samples such as blood and other species, allowing for faster reaction work than traditional modeling methods. Furthermore, the growing prevalence of chronic conditions worldwide and the growing demand for MEMS-based therapeutic procedures will create profitable opportunities for organizations in the MEMS for the therapeutic market.
Challenges:
- The introduction of new materials with novel features to complement current medical implants requires careful analysis of the impact of the materials on cellular toxicity, mutagenicity, reactivity, and stability. Moreover, replacing defective biological systems with implanted artificial equivalents must function with the same biological constraints, have consistent reliability, and ultimately show the promise of enhancing human health. Stringent regulatory guidelines imposed by government authorities and the lack of funds for setting up manufacturing utilities in the developing economies pose a major challenge for MEMS for the therapeutics market.
Market Segmentation
Segmentation Analysis of MEMS For Therapeutic Market:
- Depending on product type, the microfluidics segment is forecasted to have the highest share of the MEMS therapeutic market attributed to the development of microfluidic devices that are small in size and are termed as ""lab on chip"" systems. Microfluidics devices include microneedles, microchannels, and micro-pumps. Microneedles have several therapeutic applications. They can be employed for transdermal fluid sampling, delivery vaccines, and drug delivery. The microneedles can be utilized along with microchannels, micro-pumps, and sensors. For instance, hollow microneedles can be utilized to extract fluid from beneath the skin in a minimally invasive sampling process. The extracted fluid travels along the length of the needle and eventually into microchannels, from where it is pumped onto the active sensor surface. The sensor then detects the presence of a particular biomarker in the sample fluid. Such a process can also be incorporated in continuous glucose monitoring systems thus, consolidating the growth of the segment over the projected period.
- Depending on end-users, the home healthcare segment is projected to dominate the MEMS for therapeutic market in the forecast period. The rise in the number of individuals suffering from diabetes, cardiovascular, and hypertension is the main cause supporting the usage of MEMS devices in the home healthcare segment. MEMS technology has revolutionized diagnosing and therapeutic procedures of several diseases. The advent of microfluidics has created vast opportunities for novel detecting procedures that can help in the management and treatment of several diseases. Home healthcare services reduce the cost required for hospital visits and can give the point of care healthcare services. For instance, the continuous glucose monitoring system detects the drop in the level of insulin and stimulates the pump to inject insulin into the bloodstream. In addition, heart failure is one of the leading causes of mortality, MEMS pressure sensor can detect the changes in the blood pressure and can notify the user way before any potential risk event. Therefore, the rise in the number of wearable devices is stimulating the development of the home healthcare segment.
Regional Analysis of MEMS For Therapeutic Market:
- The North American region is anticipated to have the highest share of MEMS for the therapeutic market throughout the forecast. The early adoption of emerging technologies and the presence of prominent manufacturers of MEMS medical devices is the main factor supporting the growth of the market in this region. The growing healthcare awareness and the increasing prevalence of chronic diseases have stimulated the adoption rate of MEMS-based wearable devices. Market players in this region are investing heavily in the development of advanced self-monitoring devices such as pressure sensors. 1 out of every 4 deaths in the United States is due to a heart-related disorder. Near about 647,000 deaths occur due to heart disease, every year in the United States making it the leading cause of death. The growing prevalence of chronic diseases is fueling the growth of MEMS for the therapeutic market in this region.
- The European region is forecasted to have the second-highest share of the MEMS for the therapeutic market. The merger and acquisitions were done by major companies, and the continuous R&D are the main factors promoting the development of the MEMS for the therapeutic market. Europe is also among the top regions that are heavily affected by chronic disorders. Moreover, the supportive government policies are further propelling the growth of the MEMS for the therapeutic market in this region.
- The MEMS for the therapeutic market in the Asia-Pacific region is anticipated to develop at the highest CAGR attributed to the presence of the large semiconductors manufacturers in this region. Government and private organizations are collaborating to develop advanced MEMS for therapeutic usage. Huge investments in the Indian companies involved in the production of MEMS for healthcare services are providing a favorable condition for the expansion of the market in the Asia-Pacific region. Countries like India, China, and Japan are the prominent members contributing to the market's development.
Players Covered in MEMS For Therapeutic Market are:
- Honeywell (USA)
- Royal Philips (Netherlands)
- Texas Instruments (USA)
- STMicroelectronics (Netherlands)
- General Electric Company (USA)
- Debiotech (Switzerland)
- Agilent Technologies (USA)
- Omron Corporation (Japan)
- Silex Microsystems (Sweden)
- Sensera Ltd (Australia)
|
Global MEMS For Therapeutic Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 9.39 Bn. |
|
Forecast Period 2024-32 CAGR: |
12.7% |
Market Size in 2032: |
USD 27.54 Bn. |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: MEMS For Therapeutic Market by Type (2018-2032)
4.1 MEMS For Therapeutic Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Pressure
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Temperature
4.5 Microfluidics
4.6 Others
Chapter 5: MEMS For Therapeutic Market by Application (2018-2032)
5.1 MEMS For Therapeutic Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Hospitals
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Home Healthcare
5.5 Research Centers
5.6 Others
Chapter 6: Company Profiles and Competitive Analysis
6.1 Competitive Landscape
6.1.1 Competitive Benchmarking
6.1.2 MEMS For Therapeutic Market Share by Manufacturer (2024)
6.1.3 Industry BCG Matrix
6.1.4 Heat Map Analysis
6.1.5 Mergers and Acquisitions
6.2 KNOWLES CORPORATION (U.S.)
6.2.1 Company Overview
6.2.2 Key Executives
6.2.3 Company Snapshot
6.2.4 Role of the Company in the Market
6.2.5 Sustainability and Social Responsibility
6.2.6 Operating Business Segments
6.2.7 Product Portfolio
6.2.8 Business Performance
6.2.9 Key Strategic Moves and Recent Developments
6.2.10 SWOT Analysis
6.3 INFINEON TECHNOLOGIES AG (GERMANY)
6.4 STMICROELECTRONICS (SWITZERLAND)
6.5 AAC TECHNOLOGIES HOLDINGS INC. (CHINA)
6.6 GOERTEK INC. (CHINA)
6.7 VESPER TECHNOLOGIES INC. (U.S.)
6.8 TDK CORPORATION (JAPAN)
6.9 CIRRUS LOGIC INC. (U.S.)
6.10 INVENSENSE (PART OF TDK) (U.S.)
6.11 BOSCH SENSORTEC GMBH (GERMANY)
6.12 BSE COLTD. (SOUTH KOREA)
6.13 HOSIDEN CORPORATION (JAPAN)
6.14 MEMSENSING MICROSYSTEMS COLTD. (CHINA)
6.15 GETTOP ACOUSTIC COLTD. (CHINA)
6.16 NEW JAPAN RADIO COLTD. (JAPAN)
6.17 MOUSER ELECTRONICS INC. (U.S.)
6.18 PIEZOMIC CORPORATION (U.S.)
6.19 WOLFSON MICROELECTRONICS (CIRRUS LOGIC) (U.K.)
6.20 NEOMEMS TECHNOLOGIES INC. (U.S.)
6.21 TES ELECTRONIC SOLUTIONS GMBH (GERMANY)
Chapter 7: Global MEMS For Therapeutic Market By Region
7.1 Overview
7.2. North America MEMS For Therapeutic Market
7.2.1 Key Market Trends, Growth Factors and Opportunities
7.2.2 Top Key Companies
7.2.3 Historic and Forecasted Market Size by Segments
7.2.4 Historic and Forecasted Market Size by Type
7.2.4.1 Pressure
7.2.4.2 Temperature
7.2.4.3 Microfluidics
7.2.4.4 Others
7.2.5 Historic and Forecasted Market Size by Application
7.2.5.1 Hospitals
7.2.5.2 Home Healthcare
7.2.5.3 Research Centers
7.2.5.4 Others
7.2.6 Historic and Forecast Market Size by Country
7.2.6.1 US
7.2.6.2 Canada
7.2.6.3 Mexico
7.3. Eastern Europe MEMS For Therapeutic Market
7.3.1 Key Market Trends, Growth Factors and Opportunities
7.3.2 Top Key Companies
7.3.3 Historic and Forecasted Market Size by Segments
7.3.4 Historic and Forecasted Market Size by Type
7.3.4.1 Pressure
7.3.4.2 Temperature
7.3.4.3 Microfluidics
7.3.4.4 Others
7.3.5 Historic and Forecasted Market Size by Application
7.3.5.1 Hospitals
7.3.5.2 Home Healthcare
7.3.5.3 Research Centers
7.3.5.4 Others
7.3.6 Historic and Forecast Market Size by Country
7.3.6.1 Russia
7.3.6.2 Bulgaria
7.3.6.3 The Czech Republic
7.3.6.4 Hungary
7.3.6.5 Poland
7.3.6.6 Romania
7.3.6.7 Rest of Eastern Europe
7.4. Western Europe MEMS For Therapeutic Market
7.4.1 Key Market Trends, Growth Factors and Opportunities
7.4.2 Top Key Companies
7.4.3 Historic and Forecasted Market Size by Segments
7.4.4 Historic and Forecasted Market Size by Type
7.4.4.1 Pressure
7.4.4.2 Temperature
7.4.4.3 Microfluidics
7.4.4.4 Others
7.4.5 Historic and Forecasted Market Size by Application
7.4.5.1 Hospitals
7.4.5.2 Home Healthcare
7.4.5.3 Research Centers
7.4.5.4 Others
7.4.6 Historic and Forecast Market Size by Country
7.4.6.1 Germany
7.4.6.2 UK
7.4.6.3 France
7.4.6.4 The Netherlands
7.4.6.5 Italy
7.4.6.6 Spain
7.4.6.7 Rest of Western Europe
7.5. Asia Pacific MEMS For Therapeutic Market
7.5.1 Key Market Trends, Growth Factors and Opportunities
7.5.2 Top Key Companies
7.5.3 Historic and Forecasted Market Size by Segments
7.5.4 Historic and Forecasted Market Size by Type
7.5.4.1 Pressure
7.5.4.2 Temperature
7.5.4.3 Microfluidics
7.5.4.4 Others
7.5.5 Historic and Forecasted Market Size by Application
7.5.5.1 Hospitals
7.5.5.2 Home Healthcare
7.5.5.3 Research Centers
7.5.5.4 Others
7.5.6 Historic and Forecast Market Size by Country
7.5.6.1 China
7.5.6.2 India
7.5.6.3 Japan
7.5.6.4 South Korea
7.5.6.5 Malaysia
7.5.6.6 Thailand
7.5.6.7 Vietnam
7.5.6.8 The Philippines
7.5.6.9 Australia
7.5.6.10 New Zealand
7.5.6.11 Rest of APAC
7.6. Middle East & Africa MEMS For Therapeutic Market
7.6.1 Key Market Trends, Growth Factors and Opportunities
7.6.2 Top Key Companies
7.6.3 Historic and Forecasted Market Size by Segments
7.6.4 Historic and Forecasted Market Size by Type
7.6.4.1 Pressure
7.6.4.2 Temperature
7.6.4.3 Microfluidics
7.6.4.4 Others
7.6.5 Historic and Forecasted Market Size by Application
7.6.5.1 Hospitals
7.6.5.2 Home Healthcare
7.6.5.3 Research Centers
7.6.5.4 Others
7.6.6 Historic and Forecast Market Size by Country
7.6.6.1 Turkiye
7.6.6.2 Bahrain
7.6.6.3 Kuwait
7.6.6.4 Saudi Arabia
7.6.6.5 Qatar
7.6.6.6 UAE
7.6.6.7 Israel
7.6.6.8 South Africa
7.7. South America MEMS For Therapeutic Market
7.7.1 Key Market Trends, Growth Factors and Opportunities
7.7.2 Top Key Companies
7.7.3 Historic and Forecasted Market Size by Segments
7.7.4 Historic and Forecasted Market Size by Type
7.7.4.1 Pressure
7.7.4.2 Temperature
7.7.4.3 Microfluidics
7.7.4.4 Others
7.7.5 Historic and Forecasted Market Size by Application
7.7.5.1 Hospitals
7.7.5.2 Home Healthcare
7.7.5.3 Research Centers
7.7.5.4 Others
7.7.6 Historic and Forecast Market Size by Country
7.7.6.1 Brazil
7.7.6.2 Argentina
7.7.6.3 Rest of SA
Chapter 8 Analyst Viewpoint and Conclusion
8.1 Recommendations and Concluding Analysis
8.2 Potential Market Strategies
Chapter 9 Research Methodology
9.1 Research Process
9.2 Primary Research
9.3 Secondary Research
|
Global MEMS For Therapeutic Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 9.39 Bn. |
|
Forecast Period 2024-32 CAGR: |
12.7% |
Market Size in 2032: |
USD 27.54 Bn. |
|
Segments Covered: |
By Type |
|
|
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













