Key Market Highlights
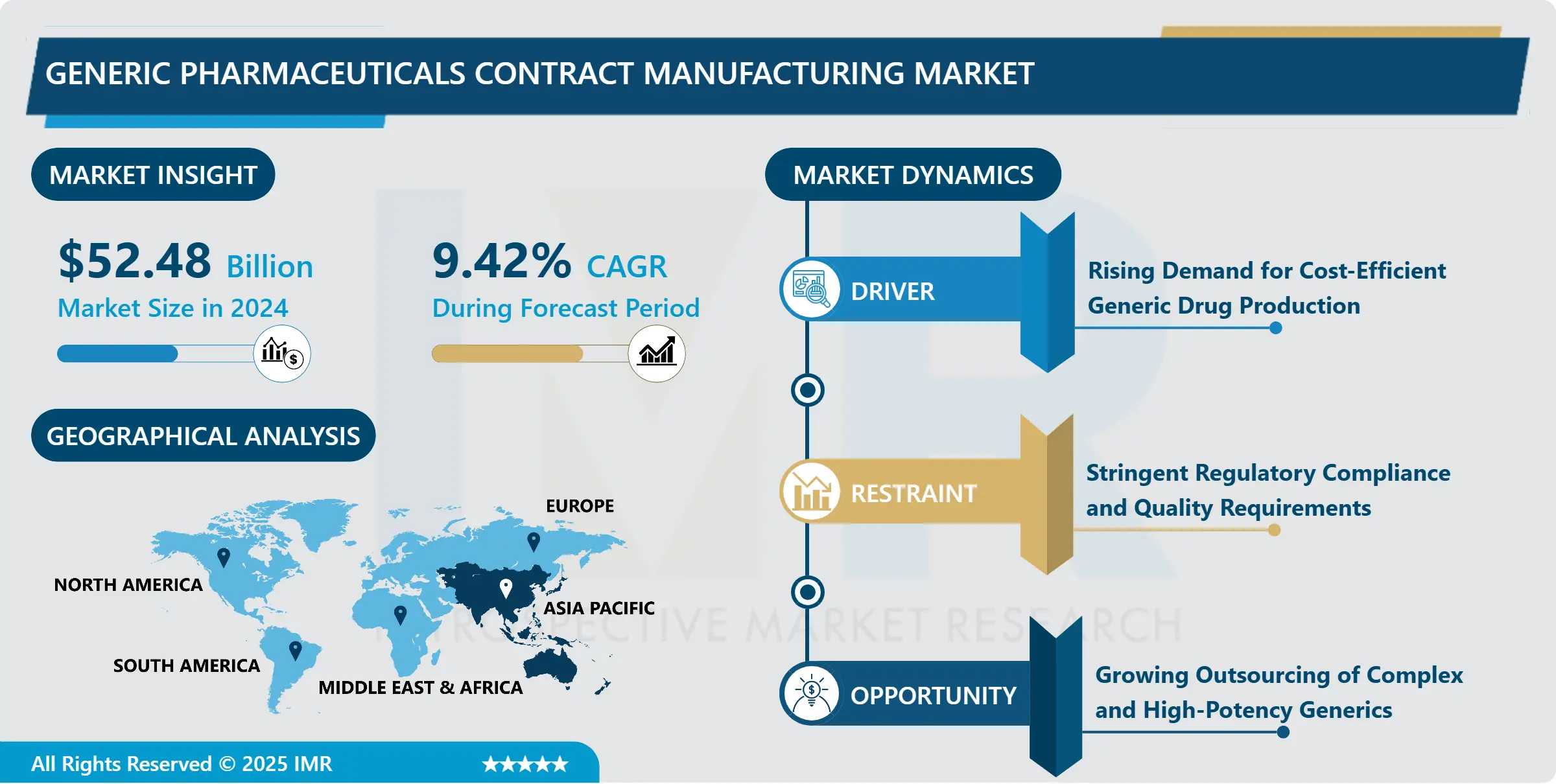
Generic Pharmaceuticals Contract Manufacturing Market Size Was Valued at USD 52.48 Billion in 2024, and is Projected to Reach USD 146.07 Billion by 2035, Growing at a CAGR of 9.42% from 2025-2035.
- Market Size in 2024: USD 52.48 Billion
- Projected Market Size by 2035: USD 146.07 Billion
- CAGR (2025–2035): 9.42%
- Leading Market in 2024: Asia-Pacific
- Fastest-Growing Market: North America
- By Drug Type: The Unbranded Generics segment is anticipated to lead the market by accounting for 56.71% of the market share throughout the forecast period.
- By Application: The Oncology segment is expected to capture 22.36% of the market share, thereby maintaining its dominance over the forecast period.
- By Region: Asia-Pacific region is projected to hold 31.52% of the market share during the forecast period.
- Active Players: Acme Generics Pvt. Ltd, (India), Alcami Corp, Inc, (United States), Aurobindo Pharma, (India), Cambrex Corp, (United States), Catalent, Inc, (United States), Curia Global, Inc, (United States), Jubilant Generics Ltd, (India), Metric Contract Services, (United States), Recipharm AB, (Sweden), Siegfried Holding AG, (Switzerland), Syngene International Ltd, (India), and Other Active Players., and Other Active Players.
Generic Pharmaceuticals Contract Manufacturing Market Synopsis:
The Generic Pharmaceuticals Contract Manufacturing Market refers to outsourcing the production of generic drugs and APIs to specialized manufacturers. The market is driven by rising demand for affordable medicines, patent expiries of branded drugs, and cost pressures on pharmaceutical companies. Growing chronic disease prevalence and the need for scalable, compliant manufacturing continue to support market demand.
Generic Pharmaceuticals Contract Manufacturing Market Dynamics and Trend Analysis:
Generic Pharmaceuticals Contract Manufacturing Market Growth Driver - Rising Demand for Cost-Efficient Generic Drug Production
-
A key growth driver of the Generic Pharmaceuticals Contract Manufacturing Market is the increasing demand for cost-efficient drug production. Healthcare systems worldwide are under pressure to reduce treatment costs while maintaining consistent drug supply. Pharmaceutical companies are therefore outsourcing manufacturing to specialized contract manufacturers that can produce high-quality generics at lower operational costs.
- Contract manufacturing enables companies to avoid heavy capital investment in facilities, comply with complex regulatory standards, and scale production quickly based on market demand. This approach is especially important for high-volume therapies such as cardiovascular, diabetes, and oncology drugs, where price sensitivity is high. As governments and insurers continue to promote affordable medicines, contract manufacturing remains a strategic solution supporting sustained market growth.
Generic Pharmaceuticals Contract Manufacturing Market Limiting Factor- Stringent Regulatory Compliance and Quality Requirements
-
A major limiting factor in the Generic Pharmaceuticals Contract Manufacturing Market is stringent regulatory and quality compliance requirements. Contract manufacturers must meet strict standards set by authorities such as the US FDA and EMA, which increases operational complexity and costs. Any compliance lapse can lead to warning letters, production delays, or loss of contracts.
- Smaller manufacturers often struggle to keep pace with evolving regulations, limiting their scalability. These challenges can slow capacity expansion and reduce flexibility, acting as a restraint on overall market growth despite strong demand for generic drugs.
Generic Pharmaceuticals Contract Manufacturing Market Expansion Opportunity- Growing Outsourcing of Complex and High-Potency Generics
-
An important expansion opportunity in the Generic Pharmaceuticals Contract Manufacturing Market lies in the growing outsourcing of complex and high-potency generic drugs. Segments such as oncology and immunology require specialized containment facilities, advanced technologies, and strict safety controls, which many pharmaceutical companies prefer not to build in-house.
- Contract manufacturers with proven expertise in high-potency APIs and complex formulations can secure long-term, high-value contracts. As more branded specialty drugs lose patent protection and healthcare systems seek affordable alternatives, demand for specialized generic manufacturing is expected to rise, creating strong expansion potential for capable contract manufacturing partners.
Generic Pharmaceuticals Contract Manufacturing Market Challenge and Risk- Margin Pressure Due to Intense Price Competition
-
One key restraint in the Generic Pharmaceuticals Contract Manufacturing Market is intense price competition, which puts continuous pressure on profit margins. Generic drug pricing is highly sensitive, driven by government tenders, bulk purchasing, and payer cost-containment policies.
- Contract manufacturers are often required to offer lower pricing while maintaining high quality and regulatory compliance, increasing operational strain. This limits investment flexibility in technology upgrades and capacity expansion. Persistent margin pressure can discourage smaller players from scaling operations and may slow overall market growth despite strong demand fundamentals.
Generic Pharmaceuticals Contract Manufacturing Market Trend- Shift Toward Integrated End-to-End Contract Manufacturing Services
-
A key trend in the Generic Pharmaceuticals Contract Manufacturing Market is the shift toward integrated, end-to-end manufacturing services. Pharmaceutical companies increasingly prefer partners that can handle API production, formulation, packaging, and regulatory support under a single contract.
- This approach reduces supply chain complexity, shortens time to market, and improves quality control. Contract manufacturers offering integrated solutions are gaining a competitive edge, particularly in high-volume and regulated generic drug segments.
Generic Pharmaceuticals Contract Manufacturing Market Segment Analysis:
Generic Pharmaceuticals Contract Manufacturing Market is segmented based on Drug Type, Application, Product Type, and Region
By Drug Type, Unbranded Generics segment is expected to dominate the market with around 56.71% share during the forecast period.
-
Within the drug type segment, unbranded generics are growing faster in the Generic Pharmaceuticals Contract Manufacturing Market. This growth is mainly driven by rising pressure on healthcare systems to reduce treatment costs and improve patient access to essential medicines. Governments, insurers, and hospital networks increasingly prefer low-cost unbranded generics, encouraging pharmaceutical companies to outsource manufacturing to achieve economies of scale.
- High volume demand, shorter commercialization timelines, and simplified branding requirements make unbranded generics well suited for contract manufacturing models. The segment is also supported by strong demand across cardiovascular, diabetes, and anti-infective therapies, particularly in emerging markets. Contract manufacturers benefit from stable, large-batch production and long-term supply agreements, making unbranded generics a key growth driver in this market.
By Application, Oncology is expected to dominate with close to 22.36% market share during the forecast period.
-
By application, the oncology segment is experiencing the strongest growth in the Generic Pharmaceuticals Contract Manufacturing Market. This growth is driven by the rising global cancer burden and the increasing adoption of generic oncology drugs as patents for branded therapies expire. Oncology treatments often require long-term therapy, creating sustained demand for cost-effective generics.
- Pharmaceutical companies prefer outsourcing oncology manufacturing to contract partners with specialized capabilities, such as high-potency API handling and regulatory compliance. The push to reduce cancer treatment costs in both developed and emerging markets further supports this trend. As healthcare systems seek affordable alternatives without compromising quality, oncology remains the most rapidly expanding application segment for contract manufacturing.
Generic Pharmaceuticals Contract Manufacturing Market Regional Insights:
Asia Pacific region is estimated to lead the market with around 31.52% share during the forecast period.
Asia Pacific is the leading region in the Generic Pharmaceuticals Contract Manufacturing Market. The region benefits from a strong manufacturing base, especially in India and China, which are global hubs for generic drug and API production. These countries offer cost-efficient manufacturing, large-scale production capacity, and a skilled technical workforce, making them preferred outsourcing destinations for global pharmaceutical companies. In addition, the presence of US FDA-approved facilities, improving regulatory compliance, and government support for pharmaceutical exports strengthen regional leadership. High demand for branded and unbranded generics, along with growing oncology, cardiovascular, and diabetes drug production, further drives market dominance. The region’s ability to deliver quality products at competitive pricing continues to attract long-term contract manufacturing partnerships.
Generic Pharmaceuticals Contract Manufacturing Market Active Players:
- Acme Generics Pvt. Ltd, (India)
- Alcami Corp, Inc, (United States)
- Aurobindo Pharma, (India)
- Cambrex Corp, (United States)
- Catalent, Inc, (United States)
- Curia Global, Inc, (United States)
- Jubilant Generics Ltd, (India)
- Metric Contract Services, (United States)
- Recipharm AB, (Sweden)
- Siegfried Holding AG, (Switzerland)
- Syngene International Ltd, (India)
- Other Active Players
Key Industry Developments in the Generic Pharmaceuticals Contract Manufacturing Market:
- In September 2024, Aurobindo Pharma has received US FDA approval for cephalexin tablets USP (250 mg and 500 mg), enhancing its generic portfolio.
- In August 2022, The acquisition of Metrics Contract Services by Catalent strengthened its oral solid dose development and manufacturing
Manufacturing Efficiency and Regulatory Precision Shaping the Generic Medicines Market
-
The generic medicines market plays a critical role in improving global access to affordable healthcare. Technically, the market is driven by the ability to deliver therapeutically equivalent drugs at significantly lower costs while maintaining strict quality and regulatory standards.
- Contract manufacturing has become a core operational model, allowing generic drug companies to outsource API production and finished dosage manufacturing to specialized partners with proven compliance records. Advances in process optimization, scale-up technologies, and quality-by-design approaches are improving production efficiency and batch consistency.
- Regulatory expertise is equally important, as generic manufacturers must meet bioequivalence requirements and comply with stringent guidelines from agencies such as the US FDA and EMA. High-volume therapeutic areas including cardiovascular, diabetes, oncology, and respiratory diseases continue to dominate manufacturing demand.
- At the same time, increasing focus on complex generics such as modified-release formulations and high-potency oncology drugs is raising the technical bar across the industry. Overall, the market is evolving toward efficient, compliant, and scalable manufacturing models that support cost control while ensuring reliable drug supply for both developed and emerging healthcare systems.
|
Generic Pharmaceuticals Contract Manufacturing Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2035 |
|
Historical Data: |
2018 to 2024 |
Market Size in 2024: |
USD 52.48 Bn. |
|
Forecast Period 2025-35 CAGR: |
9.42% |
Market Size in 2035: |
USD 146.07 Bn. |
|
Segments Covered: |
By Drug Type |
|
|
|
By Application
|
|
||
|
By Product Type |
|
||
|
By Region |
|
||
|
Growth Driver: |
|
||
|
Limiting Factor |
|
||
|
Expansion Opportunity |
|
||
|
Challenge and Risk |
|
||
|
Companies Covered in the Report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics and Opportunity Analysis
3.1.1 Growth Drivers
3.1.2 Limiting Factors
3.1.3 Growth Opportunities
3.1.4 Challenges and Risks
3.2 Market Trend Analysis
3.3 Industry Ecosystem
3.4 Industry Value Chain Mapping
3.5 Strategic PESTLE Overview
3.6 Porter's Five Forces Framework
3.7 Regulatory Framework
3.8 Pricing Trend Analysis
3.9 Intellectual Property Review
3.10 Technology Evolution
3.11 Import-Export Analysis
3.12 Consumer Behavior Analysis
3.13 Investment Pocket Analysis
3.14 Go-To Market Strategy
Chapter 4: Generic Pharmaceuticals Contract Manufacturing Market by Drug Type (2018-2035)
4.1 Generic Pharmaceuticals Contract Manufacturing Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Branded Generics and Unbranded Generics
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
Chapter 5: Generic Pharmaceuticals Contract Manufacturing Market by Product (2018-2035)
5.1 Generic Pharmaceuticals Contract Manufacturing Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Active Pharmaceutical Ingredients (APIs)
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Finished Drug Products
Chapter 6: Generic Pharmaceuticals Contract Manufacturing Market by Application (2018-2035)
6.1 Generic Pharmaceuticals Contract Manufacturing Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Oncology
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Immunology / Autoimmune
6.5 Cardiovascular
6.6 Diabetes / Metabolic
6.7 Neurology
6.8 Respiratory
6.9 Anticoagulants
6.10 Pain Management
6.11 HIV Antivirals
6.12 Others
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Generic Pharmaceuticals Contract Manufacturing Market Share by Manufacturer/Service Provider(2024)
7.1.3 Industry BCG Matrix
7.1.4 PArtnerships, Mergers & Acquisitions
7.2 ACME GENERICS PVT LTD (INDIA)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Recent News & Developments
7.2.10 SWOT Analysis
7.3 ALCAMI CORP INC (UNITED STATES)
7.4 AUROBINDO PHARMA (INDIA)
7.5 CAMBREX CORP (UNITED STATES)
7.6 CATALENT INC (UNITED STATES)
7.7 CURIA GLOBAL INC (UNITED STATES)
7.8 JUBILANT GENERICS LTD (INDIA)
7.9 METRIC CONTRACT SERVICES (UNITED STATES)
7.10 RECIPHARM AB (SWEDEN)
7.11 SIEGFRIED HOLDING AG (SWITZERLAND)
7.12 SYNGENE INTERNATIONAL LTD (INDIA)
7.13 AND OTHER ACTIVE PLAYERS.
Chapter 8: Global Generic Pharmaceuticals Contract Manufacturing Market By Region
8.1 Overview
8.2. North America Generic Pharmaceuticals Contract Manufacturing Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecast Market Size by Country
8.2.4.1 US
8.2.4.2 Canada
8.2.4.3 Mexico
8.3. Eastern Europe Generic Pharmaceuticals Contract Manufacturing Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecast Market Size by Country
8.3.4.1 Russia
8.3.4.2 Bulgaria
8.3.4.3 The Czech Republic
8.3.4.4 Hungary
8.3.4.5 Poland
8.3.4.6 Romania
8.3.4.7 Rest of Eastern Europe
8.4. Western Europe Generic Pharmaceuticals Contract Manufacturing Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecast Market Size by Country
8.4.4.1 Germany
8.4.4.2 UK
8.4.4.3 France
8.4.4.4 The Netherlands
8.4.4.5 Italy
8.4.4.6 Spain
8.4.4.7 Rest of Western Europe
8.5. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecast Market Size by Country
8.5.4.1 China
8.5.4.2 India
8.5.4.3 Japan
8.5.4.4 South Korea
8.5.4.5 Malaysia
8.5.4.6 Thailand
8.5.4.7 Vietnam
8.5.4.8 The Philippines
8.5.4.9 Australia
8.5.4.10 New Zealand
8.5.4.11 Rest of APAC
8.6. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecast Market Size by Country
8.6.4.1 Turkiye
8.6.4.2 Bahrain
8.6.4.3 Kuwait
8.6.4.4 Saudi Arabia
8.6.4.5 Qatar
8.6.4.6 UAE
8.6.4.7 Israel
8.6.4.8 South Africa
8.7. South America Generic Pharmaceuticals Contract Manufacturing Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecast Market Size by Country
8.7.4.1 Brazil
8.7.4.2 Argentina
8.7.4.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
Chapter 10 Our Thematic Research Methodology
9.1 Research Process
9.2 Primary Research
9.3 Secondary Research
Chapter 11 Case Study
Chapter 12 Appendix
10.1 Sources
10.2 List of Tables and figures
10.3 Short Forms and Citations
10.4 Assumption and Conversion
10.5 Disclaimer
|
Generic Pharmaceuticals Contract Manufacturing Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2035 |
|
Historical Data: |
2018 to 2024 |
Market Size in 2024: |
USD 52.48 Bn. |
|
Forecast Period 2025-35 CAGR: |
9.42% |
Market Size in 2035: |
USD 146.07 Bn. |
|
Segments Covered: |
By Drug Type |
|
|
|
By Application
|
|
||
|
By Product Type |
|
||
|
By Region |
|
||
|
Growth Driver: |
|
||
|
Limiting Factor |
|
||
|
Expansion Opportunity |
|
||
|
Challenge and Risk |
|
||
|
Companies Covered in the Report: |
|
||













