Pediatric Clinical Trials Market Synopsis:
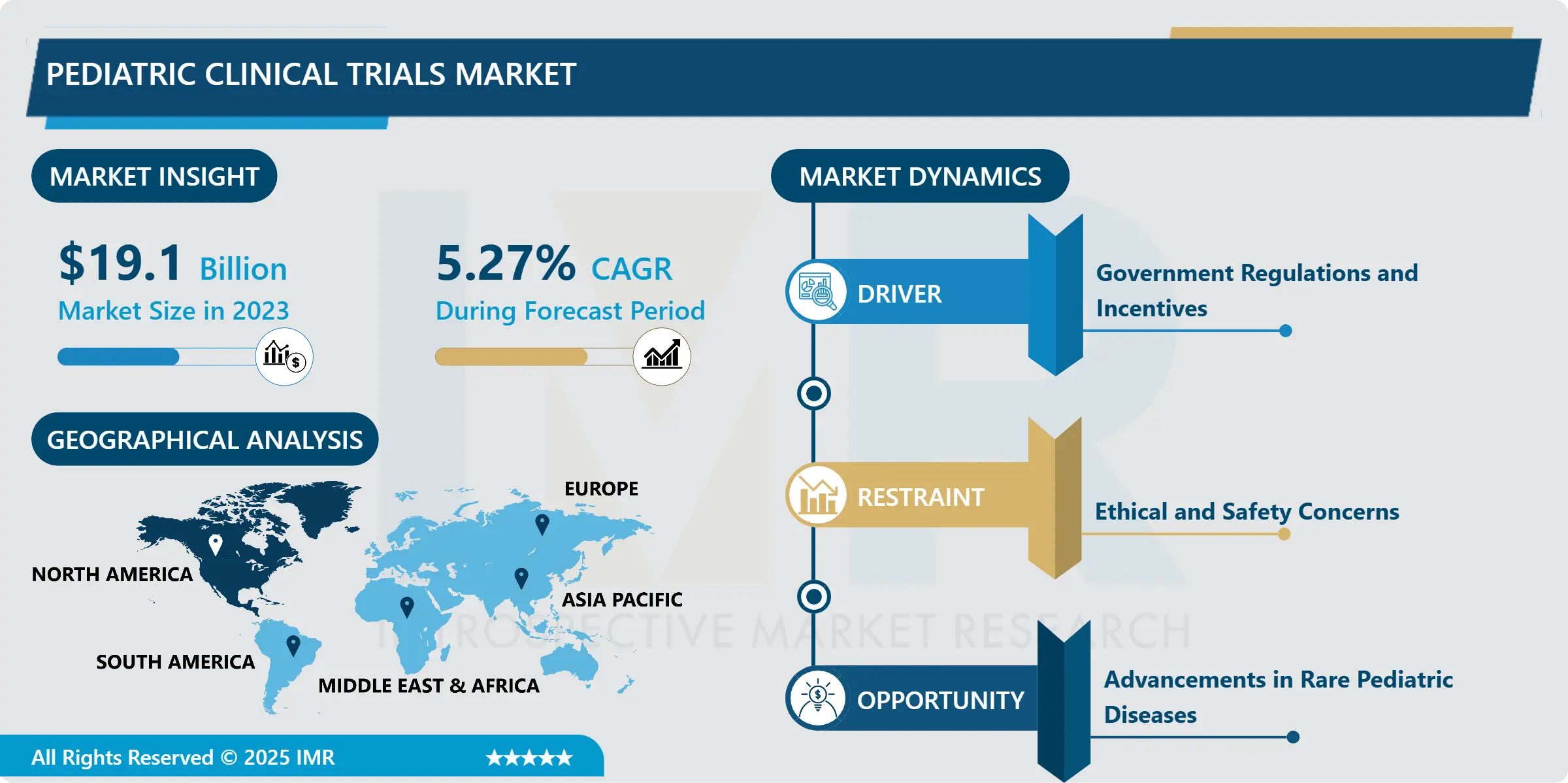
Pediatric Clinical Trials Market Size Was Valued at USD 19.10 Billion in 2023, and is Projected to Reach USD 30.32 Billion by 2032, Growing at a CAGR of 5.27% From 2024-2032.
The Pediatric Clinical Trials Market focuses on evaluating the safety, efficacy, and dosage of drugs and treatments tailored for children, including infants, children, and adolescents. These trials address unique physiological, developmental, and ethical challenges. The market has grown significantly due to rising demand for pediatric-specific treatments amid increasing cases of chronic illnesses such as asthma, diabetes, epilepsy, and emerging concerns like pediatric cancers and genetic disorders.
Pharmaceutical and biotech companies, alongside healthcare institutions, are now more invested in pediatric research. Legislation such as the U.S. Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA) mandates pediatric studies for many drugs, accelerating pediatric drug development. However, pediatric trials face substantial challenges ethical concerns about informed consent, risk exposure in children, and high costs due to specialized methodologies and monitoring.
Despite these barriers, advancements in pediatric pharmacology and increased public awareness of rare pediatric diseases have bolstered the market. Moreover, collaborations between pharmaceutical firms, universities, and regulatory bodies are enhancing trial execution and innovation. The shift toward precision medicine and a rise in pediatric drug approvals in oncology, infectious diseases, and neurology further fuel market expansion. Ultimately, coordinated efforts are vital to ensure the delivery of safe and effective treatments for children worldwide.
Pediatric Clinical Trials Market Trend Analysis:
Rise in Pediatric Oncology Trials
- A big movement that has been observed in the pediatric clinical trial market is a great increase in the pediatric oncology trials. Despite the advancement in cancer treatment over the year’s cancer continues to be among the major cause of death among children and due to this there has been improvement in designing and completion of clinical trials related to pediatric cancers. Clinical investigations in pediatric oncology are becoming more and more complex and marked by molecular targeting and genetic profiling. This trend is made possible by advances in genomics where scientists are starting to characterize which gene mutations and biomarkers are involved in children’s cancer and how drugs can be designed that would target these specific factors.
- Consequently, both pharmaceutical companies and research institutions nowadays invest more into the exploration of the topic of pediatric oncology. The recent years have shown an increased rate of approval of new drugs and therapies for pediatric cancer together with the increasing involvement of both public and private partners in more cooperation in this field. As for the segment distribution, it is expected that the growth in gene therapies and immunotherapy sectors in pediatric oncology will continue this segment’s growth. This trend is not only increasing survival rates, but also guaranteeing that children with cancer receive effective, individual treatment methods.
Advancements in Rare Pediatric Diseases
- There is much more emphasis placed on researching the less frequent pediatric diseases and conditions on the market, creating a broad opportunity for the pediatric clinical trials market. Currently, there has been more focus on rare diseases, which for a long time formed the focus of much neglect. The EU orphan medicines regulation and the U.S. Orphan Drug Act to give monetary incitements to investigate offices working with regards to rare pediatric afflictions, which have incited the treatment of rare sicknesses that were not considered viably salable previously.
- Currently, with improvements to the field of genetics and molecular biology investigations of rare diseases, and the ability to confirm new rare pediatric diseases, clinicians get an opportunity to look for cures for these diseases offered by the clinical trials. These disorders include diseases such as genetic disorders and diseases that are genetically inherited, metabolic diseases, birth defects, and other diseases that need specific management, which cannot be offered by conventional systems of medicine. An expanding pipeline of small molecule and biologic medicines, gene therapies, and enzyme replacement therapies suggests significant upside growth opportunities for pediatric clinical trials market especially as these therapies demonstrate initial signs of success in the clinical context.
Pediatric Clinical Trials Market Segment Analysis:
Pediatric Clinical Trials Market is Segmented on the basis of Phase, Therapeutic Area, Study Design, and Region.
By Phase, Preclinical segment is expected to dominate the market during the forecast period
- Clinical trials can be performed in children in different stages aimed at proven effectiveness and safety of the treatment. In pre-clinical trials scientists carry out experimental studies on animals, cellular and tissue samples as well as computer models or simulations to evaluate the drugs or devices that might be used in the actual clinical trial. It is important in this phase to try and establish any risks that may be present before moving to phase 3 that involves people. Much of the preclinical phase is very crucial in pediatrics to know how a particular treatment could be different from that on adults.
- If the treatment advances to Phase I, safety of the drug or device is checked in human beings and it involves very few study’s healthy pediatric volunteers. Phase I trials are characterized by determining drug toxicity, adverse reaction, and the right dose. Phase II trials then include a larger population to determine the efficacy, and side effects of this new drug. This sample size is important in pediatric populations where issues of thrombosis, drug metabolism, and other reasons which could have been masked in Phase I might affect outcomes. Phase III is largest with several thousands of participants to establish the efficacy of the compound and to track the safety aspect over significant period of time. Last but not the least, Phase IV are conducted after approval but are concerned with further information on the use, adverse effects, and interaction in children. All phases of pediatric clinical trials are crucial because potential treatments must be not only impactful, but safe for kids.
By Therapeutic Area, Oncology segment expected to held the largest share
- OECD countries performing pediatric clinical trials for a wide spectrum of diseases and therapeutic areas, although these trials are associated with certain challenges. Oncology requires pediatric cancer trials because childhood cancer possesses distinctive characteristics that separate it from cancer in adults. Some of them include Immunotherapy and targeted counseling which are being employed in the treatment of children cancers with better and less damaging effects. Childhood cancer clinical research is increasing as cure rates rise, and innovative therapies give children with previously incurable cancers hope.
- In neurology, the value of clinical trials involving children lies in the possibility to establish treatment regimens for various childhood diseases such as epilepsy, neurogenetic disorders or cerebral palsy. Such diseases are usually realized from childhood and they can affect the individual’s whole life hence the importance of proper treatment. The development of the application of personalized medicine and gene therapies in pediatric neurology is also giving fresh chance for therapies. Moreover, clinical trials in infectious diseases are important because the rise in antibiotic-resistant organisms and emergent viral illnesses threaten children. Concerns over increasing popularity of vaccines, antiviral agents, and immunotherapies in paediatric infectious diseases studies are aiding in overcoming these difficulties. Some other TAs like cardiovascular, respiratory and gastrointestinal diseases are also observing the growing research efforts to address pediatrics’ need.
Pediatric Clinical Trials Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America holds the largest share of the pediatric clinical trials market because of technological advancement in healthcare, a sound legal framework, and intense focus on R & D. There is in fact a vast number of pharmaceutical and biotechnology companies in the U.S. that are pro-active in the area of pediatric drugs. FDA has been very instrumental in promoting pediatric clinical trials, For Instance, the enactment of laws such as the: ‘Pediatric Research Equity Act’ and ‘The Best Pharmaceuticals for Children Act.
- The preponderance of clinical trials of pediatric Medicines in North America can also be justified by the efficiency and availability of clinical trials networks and big research clinical establishments on this region which are recognized for running most specialized pediatric clinical healthcare. Furthermore, North America has an increased awareness of the significance of pediatric clinical research specifically in diseases and pediatric cancer. This region is believed to remain dominant throughout the rest of this decade as emerging fields like gene therapy and precision medicine extend the potential of pediatric clinical trials.
Active Key Players in the Pediatric Clinical Trials Market
- AbbVie (USA)
- AstraZeneca (UK/Sweden)
- Bayer (Germany)
- Bristol-Myers Squibb (USA)
- Eli Lilly (USA)
- GlaxoSmithKline (UK)
- Johnson & Johnson (USA)
- Merck & Co. (USA)
- Novartis (Switzerland)
- Pfizer (USA)
- Roche (Switzerland)
- Sanofi (France)
- Other Active Players.
Key Industry Developments in the Pediatric Clinical Trials Market:
- In July 2024, Labcorp entered into a collaboration agreement with Naples Comprehensive Healthcare (NCH) in Southwest Florida. The collaboration aims to merge key capabilities, expertise, and technologies from both organizations to improve access to laboratory services and advance the delivery of high-quality care.
- In March 2024, Pfizer Inc. announced that the European Commission (EC) has granted marketing authorization for its 20-valent pneumococcal conjugate vaccine, known in the European Union as PREVENAR 20®. This vaccine is approved for active immunization to prevent invasive disease, pneumonia, and acute otitis media caused by Streptococcus pneumoniae in infants, children, and adolescents aged 6 weeks to under 18 years.
- In December 2023, Pfizer Inc. and Valneva SE announced the completion of participant recruitment for the Phase 3 trial, Vaccine Against Lyme for Outdoor Recreationists focused on the Lyme disease vaccine candidate VLA15. Building on positive results from earlier Phase 1 and 2 trials, this study includes both adult and pediatric participants and aims to confirm the efficacy, safety, lot consistency, and immunogenicity of VLA15.
|
Pediatric Clinical Trials Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 19.10 Billion |
|
Forecast Period 2024-32 CAGR: |
5.27% |
Market Size in 2032: |
USD 30.32 Billion |
|
Segments Covered: |
By Phase |
|
|
|
By Therapeutic Area |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Pediatric Clinical Trials Market by Distribution Channel
4.1 Pediatric Clinical Trials Market Snapshot and Growth Engine
4.2 Pediatric Clinical Trials Market Overview
4.3 Hospitals & Clinics
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 Hospitals & Clinics: Geographic Segmentation Analysis
4.4 Pharmacies & Drugstores
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Pharmacies & Drugstores: Geographic Segmentation Analysis
4.5 Online Retail
4.5.1 Introduction and Market Overview
4.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.5.3 Key Market Trends, Growth Factors and Opportunities
4.5.4 Online Retail: Geographic Segmentation Analysis
4.6 Supers & Hypers
4.6.1 Introduction and Market Overview
4.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.6.3 Key Market Trends, Growth Factors and Opportunities
4.6.4 Supers & Hypers: Geographic Segmentation Analysis
4.7 Others
4.7.1 Introduction and Market Overview
4.7.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.7.3 Key Market Trends, Growth Factors and Opportunities
4.7.4 Others: Geographic Segmentation Analysis
Chapter 5: Pediatric Clinical Trials Market by End User
5.1 Pediatric Clinical Trials Market Snapshot and Growth Engine
5.2 Pediatric Clinical Trials Market Overview
5.3 Hospitals
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Hospitals: Geographic Segmentation Analysis
5.4 Homecare
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Homecare: Geographic Segmentation Analysis
5.5 Pediatricians & Healthcare Professionals
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 Pediatricians & Healthcare Professionals: Geographic Segmentation Analysis
Chapter 6: Company Profiles and Competitive Analysis
6.1 Competitive Landscape
6.1.1 Competitive Benchmarking
6.1.2 Pediatric Clinical Trials Market Share by Manufacturer (2023)
6.1.3 Industry BCG Matrix
6.1.4 Heat Map Analysis
6.1.5 Mergers and Acquisitions
6.2 PFIZER (USA)
6.2.1 Company Overview
6.2.2 Key Executives
6.2.3 Company Snapshot
6.2.4 Role of the Company in the Market
6.2.5 Sustainability and Social Responsibility
6.2.6 Operating Business Segments
6.2.7 Product Portfolio
6.2.8 Business Performance
6.2.9 Key Strategic Moves and Recent Developments
6.2.10 SWOT Analysis
6.3 NOVARTIS (SWITZERLAND)
6.4 SANOFI (FRANCE)
6.5 ROCHE (SWITZERLAND)
6.6 JOHNSON & JOHNSON (USA)
6.7 MERCK & CO. (USA)
6.8 GLAXOSMITHKLINE (UK)
6.9 ASTRAZENECA (UK/SWEDEN)
6.10 ELI LILLY (USA)
6.11 BAYER (GERMANY)
6.12 ABBVIE (USA)
6.13 BRISTOL-MYERS SQUIBB (USA)
6.14 OTHER ACTIVE PLAYERS
Chapter 7: Global Pediatric Clinical Trials Market By Region
7.1 Overview
7.2. North America Pediatric Clinical Trials Market
7.2.1 Key Market Trends, Growth Factors and Opportunities
7.2.2 Top Key Companies
7.2.3 Historic and Forecasted Market Size by Segments
7.2.4 Historic and Forecasted Market Size By Distribution Channel
7.2.4.1 Hospitals & Clinics
7.2.4.2 Pharmacies & Drugstores
7.2.4.3 Online Retail
7.2.4.4 Supers & Hypers
7.2.4.5 Others
7.2.5 Historic and Forecasted Market Size By End User
7.2.5.1 Hospitals
7.2.5.2 Homecare
7.2.5.3 Pediatricians & Healthcare Professionals
7.2.6 Historic and Forecast Market Size by Country
7.2.6.1 US
7.2.6.2 Canada
7.2.6.3 Mexico
7.3. Eastern Europe Pediatric Clinical Trials Market
7.3.1 Key Market Trends, Growth Factors and Opportunities
7.3.2 Top Key Companies
7.3.3 Historic and Forecasted Market Size by Segments
7.3.4 Historic and Forecasted Market Size By Distribution Channel
7.3.4.1 Hospitals & Clinics
7.3.4.2 Pharmacies & Drugstores
7.3.4.3 Online Retail
7.3.4.4 Supers & Hypers
7.3.4.5 Others
7.3.5 Historic and Forecasted Market Size By End User
7.3.5.1 Hospitals
7.3.5.2 Homecare
7.3.5.3 Pediatricians & Healthcare Professionals
7.3.6 Historic and Forecast Market Size by Country
7.3.6.1 Russia
7.3.6.2 Bulgaria
7.3.6.3 The Czech Republic
7.3.6.4 Hungary
7.3.6.5 Poland
7.3.6.6 Romania
7.3.6.7 Rest of Eastern Europe
7.4. Western Europe Pediatric Clinical Trials Market
7.4.1 Key Market Trends, Growth Factors and Opportunities
7.4.2 Top Key Companies
7.4.3 Historic and Forecasted Market Size by Segments
7.4.4 Historic and Forecasted Market Size By Distribution Channel
7.4.4.1 Hospitals & Clinics
7.4.4.2 Pharmacies & Drugstores
7.4.4.3 Online Retail
7.4.4.4 Supers & Hypers
7.4.4.5 Others
7.4.5 Historic and Forecasted Market Size By End User
7.4.5.1 Hospitals
7.4.5.2 Homecare
7.4.5.3 Pediatricians & Healthcare Professionals
7.4.6 Historic and Forecast Market Size by Country
7.4.6.1 Germany
7.4.6.2 UK
7.4.6.3 France
7.4.6.4 The Netherlands
7.4.6.5 Italy
7.4.6.6 Spain
7.4.6.7 Rest of Western Europe
7.5. Asia Pacific Pediatric Clinical Trials Market
7.5.1 Key Market Trends, Growth Factors and Opportunities
7.5.2 Top Key Companies
7.5.3 Historic and Forecasted Market Size by Segments
7.5.4 Historic and Forecasted Market Size By Distribution Channel
7.5.4.1 Hospitals & Clinics
7.5.4.2 Pharmacies & Drugstores
7.5.4.3 Online Retail
7.5.4.4 Supers & Hypers
7.5.4.5 Others
7.5.5 Historic and Forecasted Market Size By End User
7.5.5.1 Hospitals
7.5.5.2 Homecare
7.5.5.3 Pediatricians & Healthcare Professionals
7.5.6 Historic and Forecast Market Size by Country
7.5.6.1 China
7.5.6.2 India
7.5.6.3 Japan
7.5.6.4 South Korea
7.5.6.5 Malaysia
7.5.6.6 Thailand
7.5.6.7 Vietnam
7.5.6.8 The Philippines
7.5.6.9 Australia
7.5.6.10 New Zealand
7.5.6.11 Rest of APAC
7.6. Middle East & Africa Pediatric Clinical Trials Market
7.6.1 Key Market Trends, Growth Factors and Opportunities
7.6.2 Top Key Companies
7.6.3 Historic and Forecasted Market Size by Segments
7.6.4 Historic and Forecasted Market Size By Distribution Channel
7.6.4.1 Hospitals & Clinics
7.6.4.2 Pharmacies & Drugstores
7.6.4.3 Online Retail
7.6.4.4 Supers & Hypers
7.6.4.5 Others
7.6.5 Historic and Forecasted Market Size By End User
7.6.5.1 Hospitals
7.6.5.2 Homecare
7.6.5.3 Pediatricians & Healthcare Professionals
7.6.6 Historic and Forecast Market Size by Country
7.6.6.1 Turkiye
7.6.6.2 Bahrain
7.6.6.3 Kuwait
7.6.6.4 Saudi Arabia
7.6.6.5 Qatar
7.6.6.6 UAE
7.6.6.7 Israel
7.6.6.8 South Africa
7.7. South America Pediatric Clinical Trials Market
7.7.1 Key Market Trends, Growth Factors and Opportunities
7.7.2 Top Key Companies
7.7.3 Historic and Forecasted Market Size by Segments
7.7.4 Historic and Forecasted Market Size By Distribution Channel
7.7.4.1 Hospitals & Clinics
7.7.4.2 Pharmacies & Drugstores
7.7.4.3 Online Retail
7.7.4.4 Supers & Hypers
7.7.4.5 Others
7.7.5 Historic and Forecasted Market Size By End User
7.7.5.1 Hospitals
7.7.5.2 Homecare
7.7.5.3 Pediatricians & Healthcare Professionals
7.7.6 Historic and Forecast Market Size by Country
7.7.6.1 Brazil
7.7.6.2 Argentina
7.7.6.3 Rest of SA
Chapter 8 Analyst Viewpoint and Conclusion
8.1 Recommendations and Concluding Analysis
8.2 Potential Market Strategies
Chapter 9 Research Methodology
9.1 Research Process
9.2 Primary Research
9.3 Secondary Research
|
Pediatric Clinical Trials Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 19.10 Billion |
|
Forecast Period 2024-32 CAGR: |
5.27% |
Market Size in 2032: |
USD 30.32 Billion |
|
Segments Covered: |
By Phase |
|
|
|
By Therapeutic Area |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













