In Vivo CRO Market Synopsis:
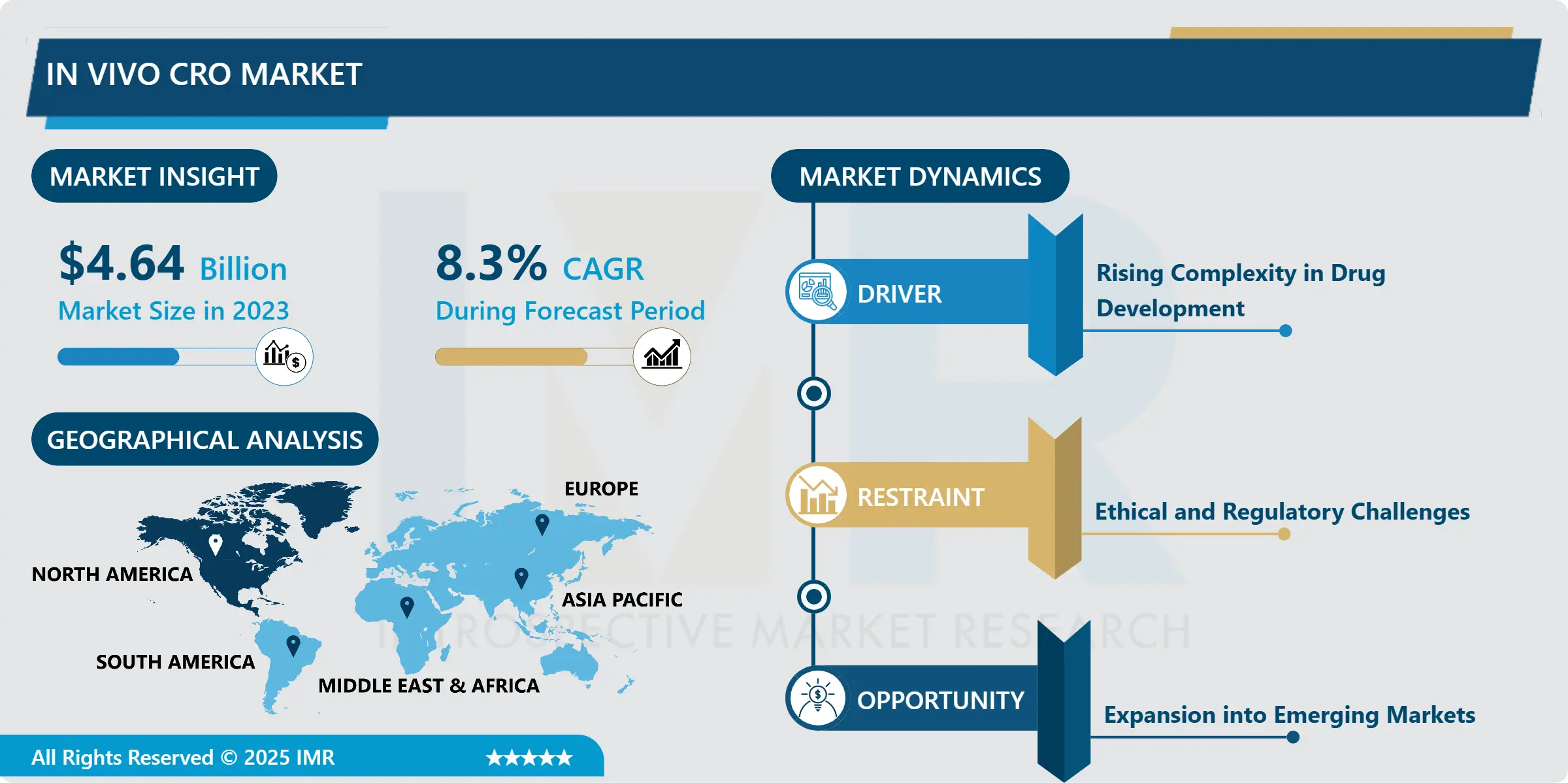
In Vivo CRO Market Size Was Valued at USD 4.64 Billion in 2023, and is Projected to Reach USD 9.51 Billion by 2032, Growing at a CAGR of 8.3% From 2024-2032.
The In Vivo Contract Research Organization (CRO) Market refers to the outsourcing services of testing involving live subjects predominantly animals in assessing safety, efficacy, and bioavailability of drugs, medical devices, and therapies. These outsourcing research services providers cater to industries including the pharma-biotech-medical device industries among others to develop, and approve products that will conform to the market requirements for their products.
The In Vivo CRO Market is very significant part of Contract Research Organization industry that offers dedicated services for preclinical research and first phase of clinical trials in the drug development process. These services allow companies to farm out time-consuming and challenging research functions to CROs, while the companies themselves apply the specialized knowledge, equipment, and software of the CRO for various in vivo studies. This market has gradually expanded its capabilities due to the need for preclinical services; regulatory demands; and the increasing expense of maintaining internal research laboratories.
The biotechnology and medical device industries also employ in vivo CROs for toxicology, kinetic and disease model testing. These organization also furthers scientific knowledge as collaborate with academic and government institutions. These biotechnological advances include gene therapies and the more recent personalized medicines; in vivo CROs play a critical role in ensuring that such advances are delivered to patients without harm or inefficiency. Moreover, preclinical data are really important for all regulatory agencies such as the FDA, and to generate such data CROs are the ideal partners for pharmaceutical companies involved in drug development.
In Vivo CRO Market Trend Analysis:
Growing Demand for Precision Medicine Studies
- With the progress of precision medicine, the market for specialized in vivo CRO has emerged correspondingly. That is why precision medicine, which means that the treatment plan also depends on a patient, requires closer consideration of medication effectiveness and potential harm at the individual level. In vivo CROs are now performing very specific experiments with genes of interest that involve the use of humanized disease animal models or PDXs. It also allows pharmaceutical and biotechnology businesses to learn about particular genetic markers, thus increasing the efficacy of their focused treatments.
- This trend is also as a result of superior technology in genomics, which is playing a big role in making of individual patient treatment. As these advances continue to move forward, in vivo CROs are also instrumental in providing the experience and resources to conduct the detailed, specialty investigations. As the field of precision medicine advances, in vivo CROs are well set to reap the benefits of increasing demand from sponsors as stakeholders in the field advance the use of even more complicated technologies and methodologies that they require to support their drug discovery work.
Expansion into Emerging Markets
- A specific growth factor that can be derived from the above analysis is that there is a great potential of the In Vivo CRO Market in Asia-Pacific and Latin America. These areas are experiencing emerging growth in pharmaceutical and biotechnology research due to the enhancement in investment, government encouragement and the growing awareness concerning health care research. Due to geographic regions’ population density and access to a cheaper pool of human capital, the vivo CROs that set up offices or affiliations in these areas will perform clinical research services at little expense.
- The opportunity is also supported by increasing governmental regulation in emerging regions adopting frameworks compatible with ICSR, simplifying the possibility for CROs to perform studies matching international standards. It provides in vivo CROs with added advantage and enable them optimally respond to increased need for clinical research services in emerging markets which are deemed to provide a healthy boost to the global candidate pipeline in the coming years.
In Vivo CRO Market Segment Analysis:
In Vivo CRO Market is Segmented on the basis of Type, Indication, End User, and Region
By Type, Rodent-based segment is expected to dominate the market during the forecast period
- The In Vivo CRO Market has been divided based on services, using rodent-based services and non-rodent-based services. Rodent based studies are the most common type as they are easy to manage, they have short life spans compared to humans, and have well developed physiology close related to humans thus appropriate for preclinical research. Toxicological efficacy and pharmacokinetic studies constitute some of the common assays they are used in and offer valid information that aids the early stages of drug development.
- However, non-rodent studies are also critical, especially when larger animals such as dogs, pigs or monkeys are used when the desired response might be closer to that observed in man. Rodent models are only sufficient in cases where simple research of easy therapeutic fields is required, while non-rodent models are more important where greater sophistication of the field of study is necessary, for example in cardiovascular or neurological investigations. This type of segmentation helps CROs to meet a range of research needs so that they can then provide a tailored service depending on the need of the study and other regulatory concerns.
By End User, Pharmaceutical Companies segment expected to held the largest share
- The primary customers of in vivo CRO services are the pharmaceutical and biotech companies and medical device manufacturers along with universities and governmental research organizations. The largest and most significant part of the market is occupied by pharmaceutical companies, which use CRO services to advance the work on the creation and development of new drugs and perform preclinical studies in accordance with existing standards. In vivo CROs are also widely used by biotechnology companies, especially in developing new therapeutic molecules including biologics, where accurate in vivo models are often required.
- In-vivo CROs help medical device firms determine the safety and efficacy of products that will be used internally including implants. Other increasing end users include academic and government research institutions since they outsource research activities to CROs. Consumer type such as pharma, biotech, medical devices, and diagnostics maintain constant demand for in vivo CRO services; however, each demands unique research and regulation services.
In Vivo CRO Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- North America Is the largest In Vivo CRO Market owing to such factors as sound pharmaceutical and biotechnology industries and well-developed research centers. It has many pharma firms that outsource some critical drug development activities to CROs in order to meet tight development timelines and FDA standards. Access to funds and talented workers also bolsters the development of in vivo CRO services, so essential for North American organizations to pursue rigorous and compliant studies.
- Also, complicated rules governing drug trials in the United States are a factor that meant that companies required the services of the CROs to meet such requirements. It also has a relatively moderate share in the market because Canada has its focus in biopharmaceutical research and the government supports life sciences. Altogether these factors build up more strength to North America to be one of the leading regions in the In Vivo CRO Market.
Active Key Players in the In Vivo CRO Market:
- Charles River Laboratories (United States)
- Covance Inc. (United States)
- ICON plc (Ireland)
- PRA Health Sciences (United States)
- Pharmaceutical Product Development (PPD) (United States)
- WuXi AppTec (China)
- Envigo (United Kingdom)
- Eurofins Scientific (Luxembourg)
- Labcorp (United States)
- Medpace (United States)
- Syneos Health (United States)
- BioAnalytical Systems, Inc. (United States)
- Other Active Players
Key Industry Developments in the In Vivo CRO Market:
- July 2024: IntegriChain, delivering pharma's comprehensive data, consulting, technology, and outsourcing platform for data-driven commercialization, acquired Federal Compliance Solutions (FCS), a leading pharma advisory and managed services firm, to develop more profitable drug commercialization strategies.
- June 2024: Lindus Health launched an all-in-one medical device CRO service, combining in-vivo CRO expertise with advanced technology and recruitment strategies, streamlining medical device clinical trials, and accelerating participant enrollment across various therapeutic areas and regulatory pathways.
- May 2024: Cannovation Clinical Research Partners launched as a CRO specializing in cannabis-based drug trials, focusing on preclinical and clinical phases, and developed cannabinoid-based therapeutics through collaborations with pharmaceutical companies, universities, and DEA researchers.
|
Global In Vivo CRO Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 4.64 Billion |
|
Forecast Period 2024-32 CAGR: |
8.3% |
Market Size in 2032: |
USD 9.51 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Indication |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: In Vivo CRO Market by Type
4.1 In Vivo CRO Market Snapshot and Growth Engine
4.2 In Vivo CRO Market Overview
4.3 Rodent-based
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 Rodent-based: Geographic Segmentation Analysis
4.4 Non-rodent-based
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Non-rodent-based: Geographic Segmentation Analysis
Chapter 5: In Vivo CRO Market by Indication
5.1 In Vivo CRO Market Snapshot and Growth Engine
5.2 In Vivo CRO Market Overview
5.3 Oncology
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Oncology: Geographic Segmentation Analysis
5.4 Autoimmune Diseases
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Autoimmune Diseases: Geographic Segmentation Analysis
5.5 Pain Management
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 Pain Management: Geographic Segmentation Analysis
5.6 Neurology
5.6.1 Introduction and Market Overview
5.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.6.3 Key Market Trends, Growth Factors and Opportunities
5.6.4 Neurology: Geographic Segmentation Analysis
5.7 Diabetes
5.7.1 Introduction and Market Overview
5.7.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.7.3 Key Market Trends, Growth Factors and Opportunities
5.7.4 Diabetes: Geographic Segmentation Analysis
5.8 Obesity
5.8.1 Introduction and Market Overview
5.8.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.8.3 Key Market Trends, Growth Factors and Opportunities
5.8.4 Obesity: Geographic Segmentation Analysis
5.9 Others
5.9.1 Introduction and Market Overview
5.9.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.9.3 Key Market Trends, Growth Factors and Opportunities
5.9.4 Others: Geographic Segmentation Analysis
Chapter 6: In Vivo CRO Market by End-User
6.1 In Vivo CRO Market Snapshot and Growth Engine
6.2 In Vivo CRO Market Overview
6.3 Pharmaceutical Companies
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Pharmaceutical Companies: Geographic Segmentation Analysis
6.4 Biotechnology Companies
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 Biotechnology Companies: Geographic Segmentation Analysis
6.5 Medical Device Companies
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 Medical Device Companies: Geographic Segmentation Analysis
6.6 Academic and Government Research Institutes
6.6.1 Introduction and Market Overview
6.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.6.3 Key Market Trends, Growth Factors and Opportunities
6.6.4 Academic and Government Research Institutes: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 In Vivo CRO Market Share by Manufacturer (2023)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 CHARLES RIVER LABORATORIES INTERNATIONAL INC.
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 EVOTEC SE
7.4 ICON PLC
7.5 IRIS PHARMA (ABIONYX PHARMA)
7.6 LABCORP DRUG DEVELOPMENT (LABORATORY CORPORATION OF AMERICA HOLDINGS)
7.7 NORTH AMERICAN SCIENCE ASSOCIATES LLC
7.8 PAREXEL INTERNATIONAL CORPORATION
7.9 PHARMACEUTICAL PRODUCT DEVELOPMENT INC. (THERMO FISHER SCIENTIFIC INC.)
7.10 PRONEXUS ANALYTICAL AB
7.11 SYNEOS HEALTH
7.12 WUXI APPTEC.
7.13 OTHER ACTIVE PLAYERS
Chapter 8: Global In Vivo CRO Market By Region
8.1 Overview
8.2. North America In Vivo CRO Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By Type
8.2.4.1 Rodent-based
8.2.4.2 Non-rodent-based
8.2.5 Historic and Forecasted Market Size By Indication
8.2.5.1 Oncology
8.2.5.2 Autoimmune Diseases
8.2.5.3 Pain Management
8.2.5.4 Neurology
8.2.5.5 Diabetes
8.2.5.6 Obesity
8.2.5.7 Others
8.2.6 Historic and Forecasted Market Size By End-User
8.2.6.1 Pharmaceutical Companies
8.2.6.2 Biotechnology Companies
8.2.6.3 Medical Device Companies
8.2.6.4 Academic and Government Research Institutes
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe In Vivo CRO Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By Type
8.3.4.1 Rodent-based
8.3.4.2 Non-rodent-based
8.3.5 Historic and Forecasted Market Size By Indication
8.3.5.1 Oncology
8.3.5.2 Autoimmune Diseases
8.3.5.3 Pain Management
8.3.5.4 Neurology
8.3.5.5 Diabetes
8.3.5.6 Obesity
8.3.5.7 Others
8.3.6 Historic and Forecasted Market Size By End-User
8.3.6.1 Pharmaceutical Companies
8.3.6.2 Biotechnology Companies
8.3.6.3 Medical Device Companies
8.3.6.4 Academic and Government Research Institutes
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe In Vivo CRO Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By Type
8.4.4.1 Rodent-based
8.4.4.2 Non-rodent-based
8.4.5 Historic and Forecasted Market Size By Indication
8.4.5.1 Oncology
8.4.5.2 Autoimmune Diseases
8.4.5.3 Pain Management
8.4.5.4 Neurology
8.4.5.5 Diabetes
8.4.5.6 Obesity
8.4.5.7 Others
8.4.6 Historic and Forecasted Market Size By End-User
8.4.6.1 Pharmaceutical Companies
8.4.6.2 Biotechnology Companies
8.4.6.3 Medical Device Companies
8.4.6.4 Academic and Government Research Institutes
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific In Vivo CRO Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By Type
8.5.4.1 Rodent-based
8.5.4.2 Non-rodent-based
8.5.5 Historic and Forecasted Market Size By Indication
8.5.5.1 Oncology
8.5.5.2 Autoimmune Diseases
8.5.5.3 Pain Management
8.5.5.4 Neurology
8.5.5.5 Diabetes
8.5.5.6 Obesity
8.5.5.7 Others
8.5.6 Historic and Forecasted Market Size By End-User
8.5.6.1 Pharmaceutical Companies
8.5.6.2 Biotechnology Companies
8.5.6.3 Medical Device Companies
8.5.6.4 Academic and Government Research Institutes
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa In Vivo CRO Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By Type
8.6.4.1 Rodent-based
8.6.4.2 Non-rodent-based
8.6.5 Historic and Forecasted Market Size By Indication
8.6.5.1 Oncology
8.6.5.2 Autoimmune Diseases
8.6.5.3 Pain Management
8.6.5.4 Neurology
8.6.5.5 Diabetes
8.6.5.6 Obesity
8.6.5.7 Others
8.6.6 Historic and Forecasted Market Size By End-User
8.6.6.1 Pharmaceutical Companies
8.6.6.2 Biotechnology Companies
8.6.6.3 Medical Device Companies
8.6.6.4 Academic and Government Research Institutes
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America In Vivo CRO Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By Type
8.7.4.1 Rodent-based
8.7.4.2 Non-rodent-based
8.7.5 Historic and Forecasted Market Size By Indication
8.7.5.1 Oncology
8.7.5.2 Autoimmune Diseases
8.7.5.3 Pain Management
8.7.5.4 Neurology
8.7.5.5 Diabetes
8.7.5.6 Obesity
8.7.5.7 Others
8.7.6 Historic and Forecasted Market Size By End-User
8.7.6.1 Pharmaceutical Companies
8.7.6.2 Biotechnology Companies
8.7.6.3 Medical Device Companies
8.7.6.4 Academic and Government Research Institutes
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Global In Vivo CRO Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 4.64 Billion |
|
Forecast Period 2024-32 CAGR: |
8.3% |
Market Size in 2032: |
USD 9.51 Billion |
|
Segments Covered: |
By Type |
|
|
|
By Indication |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













