Key Market Highlights
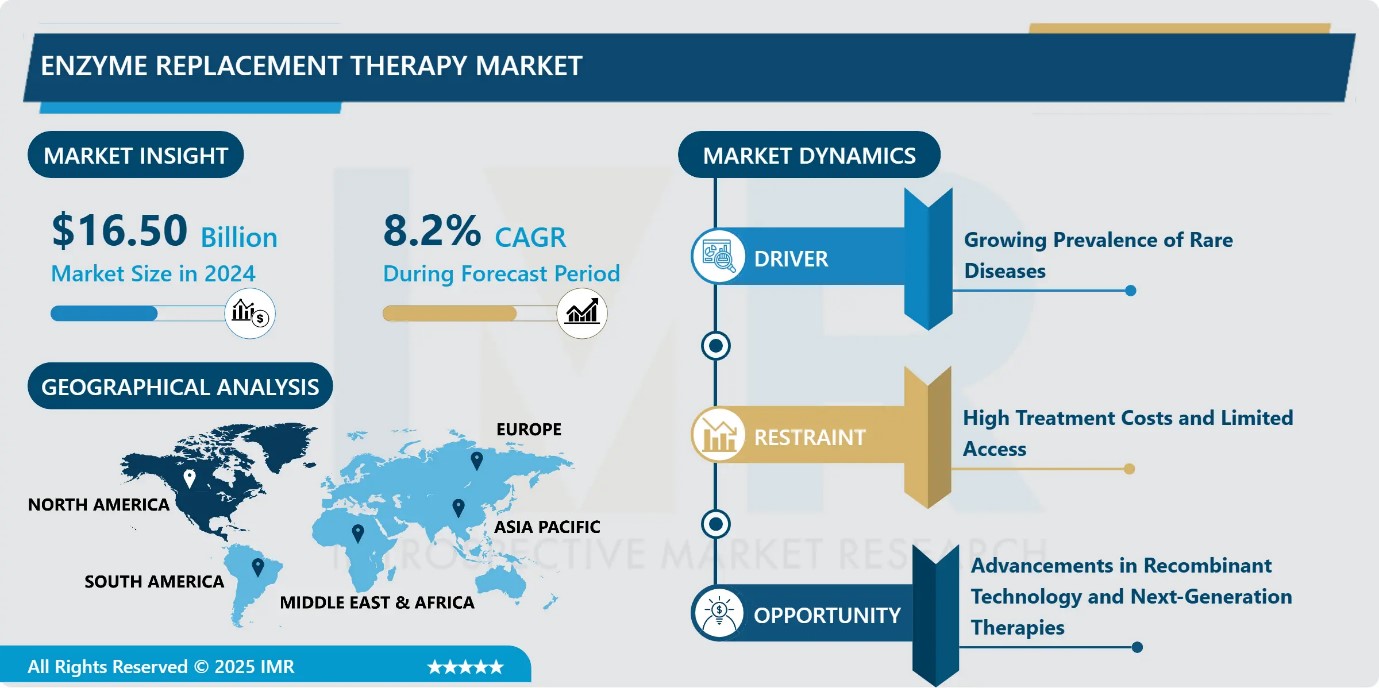
Enzyme Replacement Therapy Market Size Was Valued at USD 16.50 Billion in 2024, and is Projected to Reach USD 39.26 Billion by 2035, Growing at a CAGR of 8.2% from 2025-2035.
- Market Size in 2024: USD 16.50 Billion
- Projected Market Size by 2035: USD 39.26 Billion
- CAGR (2025–2035): 8.2%
- Leading Market in 2024: North America
- Fastest-Growing Market: Asia-Pacific
- By Product Type: The Imiglucerase segment is anticipated to lead the market by accounting for 29% of the market share throughout the forecast period.
- By Application: The Gaucher Disease segment is expected to capture 55.3% of the market share, thereby maintaining its dominance over the forecast period.
- By Region: North America region is projected to hold 31.11% of the market share during the forecast period.
- Active Players: AbbVie Inc. (U.S.), Alexion Pharmaceuticals, Inc. (U.S.), Amicus Therapeutics, Inc. (U.S.), BioMarin Pharmaceutical Inc. (U.S.), CSL Behring (Australia), and Other Active Players.
Enzyme Replacement Therapy Market Synopsis:
Enzyme Replacement Therapy (ERT) is a therapeutic approach used to treat genetic and metabolic disorders caused by the absence or deficiency of specific enzymes. It involves administering recombinant or purified enzymes to restore normal metabolic function, primarily through intravenous or other parenteral routes. The Enzyme Replacement Therapy market is witnessing steady growth due to the rising prevalence of rare genetic disorders such as Gaucher disease, Fabry disease, Pompe disease, and mucopolysaccharidoses. Improved diagnostic rates, increasing disease awareness, and advancements in biotechnology have strengthened market demand. Additionally, expanding healthcare infrastructure in emerging economies and supportive orphan drug regulations are improving patient access. Despite high treatment costs and regulatory challenges, continuous R&D investments, personalized therapies, and next-generation enzyme innovations are sustaining market momentum and long-term growth potential.
Enzyme Replacement Therapy Market Dynamics and Trend Analysis:
Enzyme Replacement Therapy Market Growth Driver-Growing Prevalence of Rare Diseases
- The increasing prevalence and improved detection of rare lysosomal storage disorders such as Gaucher, Fabry, Pompe, and MPS are key drivers of the enzyme replacement therapy market. Expanding newborn screening and genomic programs across multiple countries are identifying previously undiagnosed patient populations, enabling earlier intervention and long-term disease management.
- Early diagnosis reduces irreversible organ damage and lowers lifetime healthcare costs, encouraging payers to support early ERT adoption. Additionally, rising awareness initiatives by governments and non-profit organizations, along with favorable reimbursement frameworks in developed regions, are improving diagnosis rates and treatment accessibility. Collectively, these factors are expanding the global patient pool and driving sustained demand for enzyme replacement therapies.
Enzyme Replacement Therapy Market Limiting Factor-High Treatment Costs and Limited Access
- The enzyme replacement therapy market is constrained by the high cost of treatment and limited accessibility, particularly in developing and underinsured regions. These therapies place a substantial financial burden on healthcare systems, insurers, and patients, restricting widespread adoption.
- Differences in reimbursement coverage across countries lead to unequal patient access and fragmented demand. In emerging economies, inadequate reimbursement frameworks, limited government support, and a shortage of trained healthcare professionals often delay diagnosis and treatment initiation. While developed regions benefit from stronger insurance coverage, complex reimbursement processes and affordability concerns continue to hinder market growth, despite the critical and life-saving nature of enzyme replacement therapies.
Enzyme Replacement Therapy Market Expansion Opportunity-Advancements in Recombinant Technology and Next-Generation Therapies
- Advancements in recombinant DNA technology and gene therapy are creating significant growth opportunities in the enzyme replacement therapy market. Improved recombinant techniques enable the production of highly potent, modified enzymes with enhanced stability, targeted delivery, and reduced immunogenicity, improving therapeutic outcomes and patient safety.
- Additionally, progress in gene therapy offers the potential for long-term or curative treatment by addressing the underlying genetic defects responsible for enzyme deficiencies, potentially reducing dependence on lifelong ERT. Regulatory approvals of novel gene therapies further validate this approach. Collectively, these innovations are expanding treatment possibilities, improving patient adherence, and driving the development of next-generation ERT solutions for rare genetic disorders.
Enzyme Replacement Therapy Market Challenge and Risk-Availability of Alternatives and Immunogenicity Issues
- The enzyme replacement therapy market faces notable challenges from the emergence of alternative treatment approaches such as gene therapy, substrate reduction therapy, and stem cell transplantation, which may reduce reliance on long-term ERT. These therapies, particularly gene-based solutions, offer the potential for durable or curative outcomes, limiting ERT adoption in certain indications.
- Additionally, immunogenicity remains a significant concern, as many patients develop anti-drug antibodies that can reduce treatment efficacy or cause infusion-related reactions, leading to therapy discontinuation. Managing immune responses increases treatment complexity and costs, while strict regulatory scrutiny and limited long-term safety data for immune-mitigating technologies further constrain market growth.
Enzyme Replacement Therapy Market Trend-Expansion of Newborn Screening and Biopharmaceutical Innovation
- A key trend shaping the enzyme replacement therapy market is the growing emphasis on expanded newborn screening programs for early detection of metabolic and enzyme-deficiency disorders. Early identification enables the timely initiation of ERT, preventing irreversible organ damage and significantly improving long-term outcomes.
- Governments and health agencies, including the NIH, are supporting the inclusion of additional rare conditions in national screening panels, increasing the diagnosed patient pool. Simultaneously, advancements in biopharmaceutical technologies, such as recombinant DNA techniques and protein engineering, are leading to the development of safer, more effective enzymes and improved delivery systems. These innovations are accelerating new product launches, attracting investment, and enhancing treatment accessibility.
Enzyme Replacement Therapy Market Segment Analysis:
Enzyme Replacement Therapy Market is segmented based on Drug Class, Product Type, Application, Therapeutic Area, Formulation Type, Patient Age Group, End User, and Region.
By Product Type, Imiglucerase segment is expected to dominate the market with around 29% share during the forecast period.
- Imiglucerase continues to dominate the enzyme replacement therapy market, driven by its long-standing clinical use and proven efficacy in treating Type 1 Gaucher disease. Its strong safety profile, broad approval across adult and pediatric populations, and extensive physician familiarity have established it as a standard of care.
- The increasing diagnosis of Gaucher disease further supports sustained demand for imiglucerase-based therapies. However, the segment faces growing competition from next-generation enzyme therapies designed with improved lysosomal targeting, extended half-life, and reduced immunogenicity. While these innovations are gaining traction, imiglucerase remains dominant due to its established clinical outcomes, regulatory approvals, and widespread adoption across global treatment centers.
By Application, Gaucher disease is expected to dominate with close to 55.3% market share during the forecast period.
- The Gaucher disease segment led the global enzyme replacement therapy market in 2024, supported by its relatively higher prevalence among lysosomal storage disorders and the availability of multiple approved ERT products. Well-established treatment protocols, standardized dosing regimens, and long-term clinical evidence have made ERT a cornerstone in Gaucher disease management. Improved diagnostic capabilities, increased disease awareness, and timely treatment initiation further contribute to segment growth.
- Ongoing research and continuous patient monitoring strengthen treatment outcomes and adoption rates. Although emerging innovations in Pompe and Fabry disease therapies are gaining momentum, Gaucher disease remains dominant due to its mature therapeutic landscape, proven efficacy of existing enzymes, and strong physician confidence in ERT-based treatment approaches.
Enzyme Replacement Therapy Market Regional Insights:
North America region is estimated to lead the market with around 31.11% share during the forecast period.
- North America dominated the global enzyme replacement therapy market, accounting for approximately 31.11% of revenue in 2024, driven by a high prevalence of rare genetic and lysosomal storage disorders and a well-established healthcare infrastructure. Strong investments in research and development, extensive clinical trial activity, and frequent new product approvals by the U.S. FDA support continuous innovation.
- Favorable reimbursement policies, broad insurance coverage, and newborn screening programs enable early diagnosis and long-term treatment access. The region is dominant due to the presence of leading pharmaceutical companies, advanced regulatory support, specialized treatment centers, and high healthcare spending that ensures rapid adoption of novel ERT therapies.
Enzyme Replacement Therapy Market Active Players:
- AbbVie Inc. (U.S.)
- Alexion Pharmaceuticals, Inc. (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- CSL Behring (Australia)
- GC Biopharma (South Korea)
- Horizon Therapeutics plc (Ireland)
- JCR Pharmaceuticals Co., Ltd. (Japan)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Protalix BioTherapeutics, Inc. (Israel)
- Recordati Rare Diseases (Italy)
- Sanofi (Sanofi Genzyme) (France)
- Takeda Pharmaceutical Company Limited (Japan)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Other Active Players
Key Industry Developments in the Enzyme Replacement Therapy Market:
- In March 2025, Sanofi received FDA approval for Qfitlia (fitusiran), marking a major advancement in hemophilia care. Qfitlia is the first antithrombin-lowering therapy approved for the treatment of hemophilia A and B, offering a novel mechanism of action. The therapy significantly reduces treatment burden by requiring only six injections per year, improving patient adherence and quality of life.
- In March 2025, Ultragenyx reported strong financial performance, posting Q1 2025 revenues of USD 139 million, reflecting continued demand for its rare disease portfolio. The company also achieved a key regulatory milestone as its investigational gene therapy, UX111, developed for the treatment of Sanfilippo syndrome, received an FDA PDUFA action date of August 18, 2025. This development highlights Ultragenyx’s expanding focus on advanced gene therapies for rare and life-threatening genetic disorders.
Technical Overview and Mechanistic Insights of Enzyme Replacement Therapy (ERT) in Treating Rare Genetic and Metabolic Disorders
- Enzyme Replacement Therapy (ERT) involves administering exogenous enzymes to patients with inherited or acquired enzyme deficiencies to restore normal metabolic function. ERT primarily uses recombinant or purified human enzymes, delivered via intravenous infusion, subcutaneous injection, or other parenteral routes. Advances in recombinant DNA technology, protein engineering, and glyco-modification techniques have enhanced enzyme stability, lysosomal targeting, and reduced immunogenicity.
- ERT targets lysosomal storage disorders, glycogen storage diseases, and other metabolic conditions, enabling substrate degradation and mitigating organ damage. Ongoing research into next-generation ERTs and gene therapies aims to improve pharmacokinetics, reduce dosing frequency, and increase patient adherence, expanding therapeutic options for rare genetic disorders.
|
Enzyme Replacement Therapy Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2035 |
|
Historical Data: |
2018 to 2023 |
Market Size in 2024: |
USD 16.50 Bn. |
|
Forecast Period 2025-32 CAGR: |
8.2 % |
Market Size in 2035: |
USD 39.26 Bn. |
|
Segments Covered: |
By Drug Class |
|
|
|
By Product Type |
|
||
|
By Application
|
|
||
|
By Therapeutic Area |
|
||
|
By Formulation Type |
|
||
|
By Patient Age Group |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Growth Driver: |
|
||
|
Limiting Factor |
|
||
|
Expansion Opportunity |
|
||
|
Challenge and Risk |
|
||
|
Companies Covered in the Report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics and Opportunity Analysis
3.1.1 Growth Drivers
3.1.2 Limiting Factors
3.1.3 Growth Opportunities
3.1.4 Challenges and Risks
3.2 Market Trend Analysis
3.3 Industry Ecosystem
3.4 Industry Value Chain Mapping
3.5 Strategic PESTLE Overview
3.6 Porter's Five Forces Framework
3.7 Regulatory Framework
3.8 Pricing Trend Analysis
3.9 Intellectual Property Review
3.10 Technology Evolution
3.11 Import-Export Analysis
3.12 Consumer Behavior Analysis
3.13 Investment Pocket Analysis
3.14 Go-To Market Strategy
Chapter 4: Enzyme Replacement Therapy Market by Drug Class (2018-2035)
4.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Alglucosidase Alfa
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Agalsidase
4.5 Pancrelipase
4.6 Idursulfase
4.7 Laronidase
4.8 Imiglucerase
4.9 Elosulfase Alfa
4.10 Asfotase Alfa
4.11 Galsulfase
4.12 Velaglucerase Alfa
4.13 and Others
Chapter 5: Enzyme Replacement Therapy Market by Product Type (2018-2035)
5.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Imiglucerase
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Agalsidase Beta
5.5 Taliglucerase
5.6 Velaglucerase Alfa
5.7 and Others
Chapter 6: Enzyme Replacement Therapy Market by Application (2018-2035)
6.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Gaucher Disease (Type I
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 II
6.5 III)
6.6 Fabry Disease
6.7 Pompe Disease
6.8 Mucopolysaccharidosis (MPS)
6.9 and Others
Chapter 7: Enzyme Replacement Therapy Market by Therapeutic Area (2018-2035)
7.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
7.2 Market Overview
7.3 Metabolic Disorders
7.3.1 Introduction and Market Overview
7.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
7.3.3 Key Market Trends, Growth Factors, and Opportunities
7.3.4 Geographic Segmentation Analysis
7.4 Glycogen Storage Diseases
7.5 Lysosomal Storage Disorders
7.6 Neurodegenerative Disorders
7.7 and Others
Chapter 8: Enzyme Replacement Therapy Market by Formulation Type (2018-2035)
8.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
8.2 Market Overview
8.3 Liquid Formulation
8.3.1 Introduction and Market Overview
8.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
8.3.3 Key Market Trends, Growth Factors, and Opportunities
8.3.4 Geographic Segmentation Analysis
8.4 Lyophilized Powder
8.5 and Others
Chapter 9: Enzyme Replacement Therapy Market by Patient Age Group (2018-2035)
9.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
9.2 Market Overview
9.3 Pediatric and Adult
9.3.1 Introduction and Market Overview
9.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
9.3.3 Key Market Trends, Growth Factors, and Opportunities
9.3.4 Geographic Segmentation Analysis
Chapter 10: Enzyme Replacement Therapy Market by End User (2018-2035)
10.1 Enzyme Replacement Therapy Market Snapshot and Growth Engine
10.2 Market Overview
10.3 Hospitals
10.3.1 Introduction and Market Overview
10.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
10.3.3 Key Market Trends, Growth Factors, and Opportunities
10.3.4 Geographic Segmentation Analysis
10.4 Infusion Centers
10.5 Homecare Settings
10.6 and Others
Chapter 11: Company Profiles and Competitive Analysis
11.1 Competitive Landscape
11.1.1 Competitive Benchmarking
11.1.2 Enzyme Replacement Therapy Market Share by Manufacturer/Service Provider(2024)
11.1.3 Industry BCG Matrix
11.1.4 PArtnerships, Mergers & Acquisitions
11.2 ABBVIE INC. (U.S.)
11.2.1 Company Overview
11.2.2 Key Executives
11.2.3 Company Snapshot
11.2.4 Role of the Company in the Market
11.2.5 Sustainability and Social Responsibility
11.2.6 Operating Business Segments
11.2.7 Product Portfolio
11.2.8 Business Performance
11.2.9 Recent News & Developments
11.2.10 SWOT Analysis
11.3 ALEXION PHARMACEUTICALS
11.4 INC. (U.S.)
11.5 AMICUS THERAPEUTICS
11.6 INC. (U.S.)
11.7 BIOMARIN PHARMACEUTICAL INC. (U.S.)
11.8 CSL BEHRING (AUSTRALIA)
11.9 GC BIOPHARMA (SOUTH KOREA)
11.10 HORIZON THERAPEUTICS PLC (IRELAND)
11.11 JCR PHARMACEUTICALS CO.
11.12 LTD. (JAPAN)
11.13 NOVARTIS AG (SWITZERLAND)
11.14 PFIZER INC. (U.S.)
11.15 PROTALIX BIOTHERAPEUTICS
11.16 INC. (ISRAEL)
11.17 RECORDATI RARE DISEASES (ITALY)
11.18 SANOFI (SANOFI GENZYME) (FRANCE)
11.19 TAKEDA PHARMACEUTICAL COMPANY LIMITED (JAPAN)
11.20 ULTRAGENYX PHARMACEUTICAL INC. (U.S.)
11.21 AND OTHER ACTIVE PLAYERS.
Chapter 12: Global Enzyme Replacement Therapy Market By Region
12.1 Overview
12.2. North America Enzyme Replacement Therapy Market
12.2.1 Key Market Trends, Growth Factors and Opportunities
12.2.2 Top Key Companies
12.2.3 Historic and Forecasted Market Size by Segments
12.2.4 Historic and Forecast Market Size by Country
12.2.4.1 US
12.2.4.2 Canada
12.2.4.3 Mexico
12.3. Eastern Europe Enzyme Replacement Therapy Market
12.3.1 Key Market Trends, Growth Factors and Opportunities
12.3.2 Top Key Companies
12.3.3 Historic and Forecasted Market Size by Segments
12.3.4 Historic and Forecast Market Size by Country
12.3.4.1 Russia
12.3.4.2 Bulgaria
12.3.4.3 The Czech Republic
12.3.4.4 Hungary
12.3.4.5 Poland
12.3.4.6 Romania
12.3.4.7 Rest of Eastern Europe
12.4. Western Europe Enzyme Replacement Therapy Market
12.4.1 Key Market Trends, Growth Factors and Opportunities
12.4.2 Top Key Companies
12.4.3 Historic and Forecasted Market Size by Segments
12.4.4 Historic and Forecast Market Size by Country
12.4.4.1 Germany
12.4.4.2 UK
12.4.4.3 France
12.4.4.4 The Netherlands
12.4.4.5 Italy
12.4.4.6 Spain
12.4.4.7 Rest of Western Europe
12.5. Asia Pacific Enzyme Replacement Therapy Market
12.5.1 Key Market Trends, Growth Factors and Opportunities
12.5.2 Top Key Companies
12.5.3 Historic and Forecasted Market Size by Segments
12.5.4 Historic and Forecast Market Size by Country
12.5.4.1 China
12.5.4.2 India
12.5.4.3 Japan
12.5.4.4 South Korea
12.5.4.5 Malaysia
12.5.4.6 Thailand
12.5.4.7 Vietnam
12.5.4.8 The Philippines
12.5.4.9 Australia
12.5.4.10 New Zealand
12.5.4.11 Rest of APAC
12.6. Middle East & Africa Enzyme Replacement Therapy Market
12.6.1 Key Market Trends, Growth Factors and Opportunities
12.6.2 Top Key Companies
12.6.3 Historic and Forecasted Market Size by Segments
12.6.4 Historic and Forecast Market Size by Country
12.6.4.1 Turkiye
12.6.4.2 Bahrain
12.6.4.3 Kuwait
12.6.4.4 Saudi Arabia
12.6.4.5 Qatar
12.6.4.6 UAE
12.6.4.7 Israel
12.6.4.8 South Africa
12.7. South America Enzyme Replacement Therapy Market
12.7.1 Key Market Trends, Growth Factors and Opportunities
12.7.2 Top Key Companies
12.7.3 Historic and Forecasted Market Size by Segments
12.7.4 Historic and Forecast Market Size by Country
12.7.4.1 Brazil
12.7.4.2 Argentina
12.7.4.3 Rest of SA
Chapter 13 Analyst Viewpoint and Conclusion
Chapter 14 Our Thematic Research Methodology
14.1 Research Process
14.2 Primary Research
14.3 Secondary Research
Chapter 15 Case Study
Chapter 16 Appendix
16.1 Sources
16.2 List of Tables and figures
16.3 Short Forms and Citations
16.4 Assumption and Conversion
16.5 Disclaimer
|
Enzyme Replacement Therapy Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2035 |
|
Historical Data: |
2018 to 2023 |
Market Size in 2024: |
USD 16.50 Bn. |
|
Forecast Period 2025-32 CAGR: |
8.2 % |
Market Size in 2035: |
USD 39.26 Bn. |
|
Segments Covered: |
By Drug Class |
|
|
|
By Product Type |
|
||
|
By Application
|
|
||
|
By Therapeutic Area |
|
||
|
By Formulation Type |
|
||
|
By Patient Age Group |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Growth Driver: |
|
||
|
Limiting Factor |
|
||
|
Expansion Opportunity |
|
||
|
Challenge and Risk |
|
||
|
Companies Covered in the Report: |
|
||













