Metastatic Castrate Resistant Prostate Cancer Treatment Market Synopsis
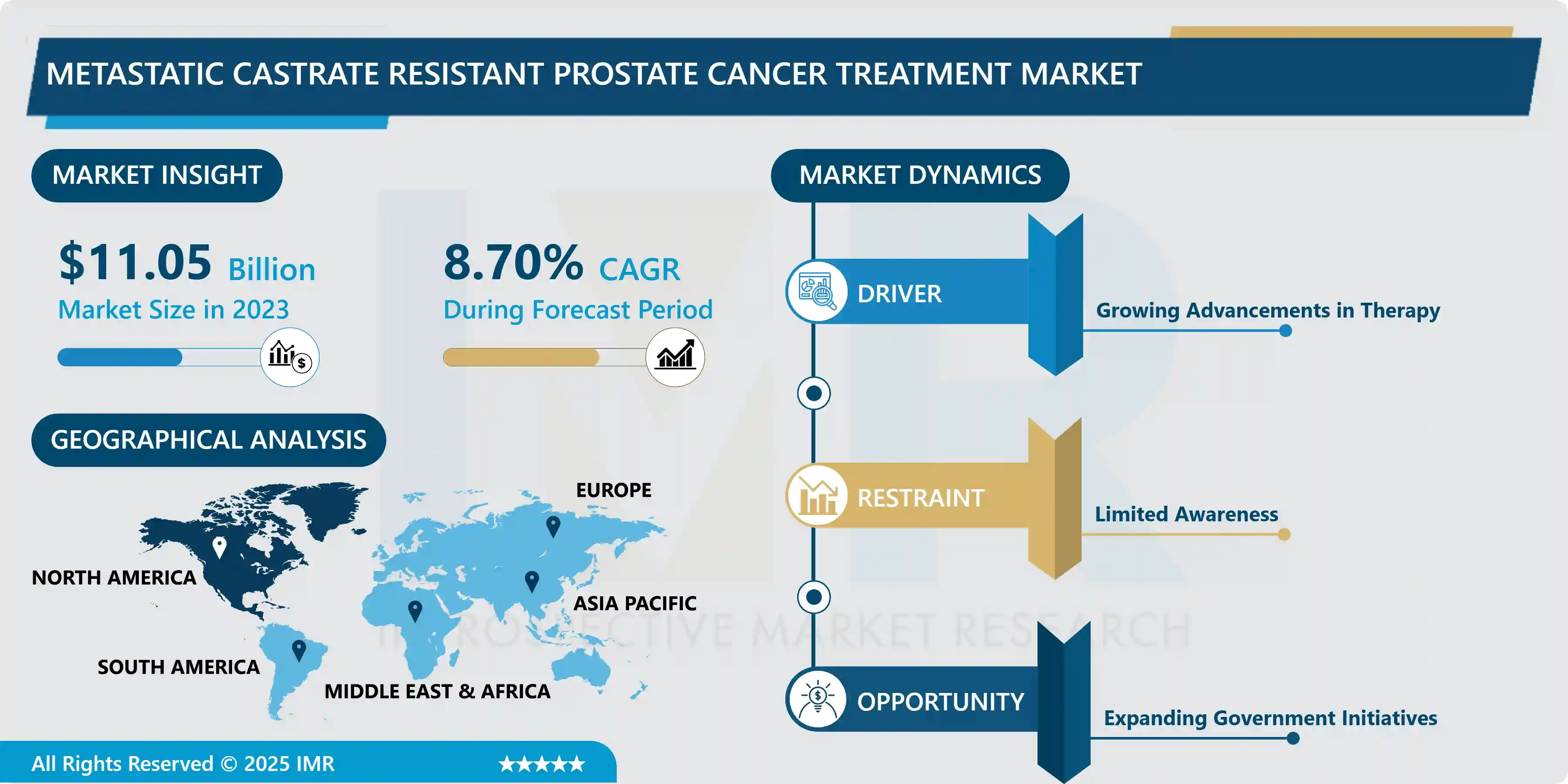
Metastatic Castrate Resistant Prostate Cancer Treatment Market Size Was Valued at USD 11.05 Billion in 2023, and is Projected to Reach USD 23.41 Billion by 2032, Growing at a CAGR of 8.7% From 2024-2032.
The Metastatic Castrate Resistant Prostate Cancer (mCRPC) Treatment Market is defined as the global industry encompassing products and services provided to patients with metastatic prostate cancer who have had poor response to androgen deprivation therapy (ADT). Since mCRPC is a metastatic form of prostate cancer that has progressed through disease progression while being treated with medical or surgical castration, the standard treatment option use novel hormonal therapies, chemotherapy, immunotherapy as well as radiopharmaceutical. The market includes the products that are developed concerning androgen receptor signaling, bone management and inherent susceptibilities in the cancers cells.
The metastatic castrate-resistant prostate cancer (mCRPC) treatment market has evolved greatly on account of a rising incidence of prostate cancer and continued research into new therapies. , Instead, for patients developing metastatic prostate cancer, the option changes to more aggressive treatment that involves hormonal therapy, chemotherapy, and novel molecular therapies. In particular, drugs such as abiraterone acetate and enzalutamide are currently used as androgen receptor inhibitors, which significantly changed the patients’ prognosis by providing more adequate opportunities for treating the disease and inhibit its progression; as a result, the market demand has increased. Furthermore, the knowledge of exciting new treatments like radioligand therapy and immunotherapy is extending the options for many patients with few other alternative treatment choices.
In the geographical segmentation, North America dominates the mCRPC treatment market because of high healthcare spending, better research activities and better approval system for potential treatment. But the Asia-Pacific region is believed to show a higher growth rate in the installation of abiraterone because of awareness, enhanced healthcare structures, and treatment availability. There are giants in the market like Johnson & Johnson, Astellas Pharma and Bayer actively involved in the R & D activities for the introduction of subsequent state of art therapeutics. In addition, there are several production companies ,research institutions that are developing partnerships that are encouraging the development of new products that add on to the available treatment programs for mCRPC patients.
Market obstacles comprise high treatment costs and the possibility of the development of negative dependencies toward therapies, requiring further works on combination therapies and biomarker approach. Also growth can be affected by the changing regulatory rules and reimbursement systems, among others. However, considerable future growth is expected in the mCRPC treatment market due to the new pipeline products which signify better survival and quality of life among patients. The encouraging of the personalized medicine and the true incorporation of the advanced diagnostics are thought to continue improving the management plan, at last, in t?i contribute to the benefits of patient in this daunting disease setting.

Metastatic Castrate Resistant Prostate Cancer Treatment Market Trend Analysis
Growing Adoption of Novel Androgen Receptor Inhibitors and Targeted Therapies in mCRPC Treatment
- The latest trend apparent in the mCRPC treatment market is the frequent utilization of more selective androgen receptor inhibitors like enzalutamide and apalutamide. These inhibitors have demonstrated effectiveness in increasing progression-free survival and the survival rates of patients with metastatic prostate cancer who are no longer benefited by standard androgen ablation treatments. Unlike previous drugs, the new medicines are stronger in binding the androgen receptors and thus restrict the ability of cancer cells to use testosterone, a fuel to prostate cancer. This ability to provide a more complete block of the androgen receptor pathway has made these drugs the mainstay of the management of prostate cancer that has metastasized. Advancements in next-generation androgen receptor inhibitors in terms of safety and efficacy characteristics increases the applicability of these drugs for treating populations with high risk disease features.
- Furthermore, PARP inhibitors could be viewed as another breakthrough in targeted therapies as part of mCRPC treatment for patient’s with DNA repair genes BRCA1/2 alterations . PARP inhibitors, including olaparib and rucaparib act selectively to target cancerous cells orally with defective DNA repair based procedures thereby negating the harms on the normal cells. This individualization is especially appealing since it severs the one-size-fits-all treatment strategy by providing prescription specifics according to the genes of a patient and thereby introducing the theory of precision medicine in prostate cancer treatment. These therapies have been proven to have high levels of effectiveness in trials with regards to surving progression free survival of patients with high genotypes. Through achieving a direct correlation between genetic testing and biomarker analysis integrated with treatment, physicians are now able to personalise patient’s treatment needs which is a major revolution in the management of the mCRPC and gives credence to the role of personalised strategies.
Integration of Immuno-Oncology Therapies and Radiopharmaceuticals in mCRPC Treatment
- Yet another major factor driving change in the mCRPC treatment market is the ongoing convergence of immuno-oncology agents. Of these, the checkpoint inhibitors especially the PD-1 and PD-L1 inhibitors are wearing more confidence in clinical trial environment as an adjuvant to the existing therapies. These immunotherapies act through boosting the immune response to cancer cells and this can be described as ‘vigorously awakening’ the immune system.While both are still considered experimental, preliminary studies hint that these immunotherapies, when used a in combination with other conventional therapies, like androgen receptor inhibitors or chemotherapy, could increase survival rates and decrease the size of tumors. These promising combinations show an increasing trend in the orientation to more complex treatment strategies that stimulate the body’s immune system, making immunotherapy a major focus of current research and clinical practice.
- Similarly, novel systemic agents like Radium-223 and Lutetium-177 are rapidly expanding the menu of therapeutic options available to mCRPC patients especially if they have bone metastases. These agents gives a precise doses of radiation to those cancer cells, it also relieves bone pain and controls tumor growth with little destructive impact on of healthy tissues. For example, the versed Radium-223 targets bone metastases, Lutetium-177 may be of potential use for patients with neuroendocrine features, or those overexpressing PSMA. This novel approach goes a long way in strengthening treatment outcomes while extending possibilities of disease complexity directions in mCRPC. In combination with the constant further development of new drugs such as new compound drug combinations and individual therapy programs, these achievements show a shift in static therapy, focusing on enhanced patient survival and quality of life in this difficult disease environment.
Metastatic Castrate Resistant Prostate Cancer Treatment Market Segment Analysis:
Metastatic Castrate Resistant Prostate Cancer Treatment Market Segmented based on By Treatment, By Route of Administration and By End-Users
By Treatment, Hormone Therapies segment is expected to dominate the market during the forecast period
- Aromatase inhibitors are also invaluable during the treatment of hormone receptor-positive breast cancer because they reduce the production of estrogen that fuels the diseased tissue growth. These therapies employ a wide range of agents such as luteinizing hormone-releasing hormone (LHRH) agonists and antagonists which interfere with the paths in the cell that the cancer cells use in their growth. These treatments will reduce the masculine hormone thereby reducing the rate at which the disease progresses, and the size of the tumor or its ability to spread will be greatly reduced. In doing so, this therapeutic modality not only increases survival probabilities but also improves patient’s currency by diminishing suffering often characteristic of later stages of malignant neoplasia. Therefore, hormone therapies are part and parcel of the management protocols for prostate cancer and other hormone-responsive cancers.
- As the rate of hormone-sensitive cancer rises, significant efforts have been dedicated to generate and develop potential therapeutic intervention and combination therapies improving the efficacy. Significant current research is being dedicated to trying to establish the use of hormone therapies in combination with immunotherapy and targeted therapies. Furthermore, there are improvements in the specificity of the ‘principle of molecular targeting’ in that directions are being made towards personalizing the choice of hormones, based on patient characteristics and tumor characteristics. Such changes in treatment approaches has been attributable to advancements in cancer therapy and practice whereby this and other types of cancer will continue to see enhancements in further innovations that will actually address the issue and help patients live longer, comfortable lives with less side effects. In future, more hormonal therapies will be discovered as patients diagnosed with hormone-sensitive cancers seek for treatment.
By End-Users, Hospitals segment held the largest share in 2023
- Hospitals are the main actors in the delivery of cancer care because they can accommodate many difficult and complicated cases, which more often need elaborate treatment. Currently, advanced diagnostic products like imaging and biomarkers screening help hospitals determine the nature and progression of cancer to guide oncologists in developing appropriate treatment strategies. Each patient is developed an individual treatment plan after interaction of a group of professionals, including surgeons, medical oncologists, radiation oncologists and nursing staff. It also makes it possible for the hospitals to provide the complete spectrum of cancer treatment options including conventional chemotherapeutic agents and radiotherapy to novel immunotherapies, and targeted therapies. Therefore, the hospitals are not only the places where the cured are given treatments but also remarkable organizations involved in research and clinical trials in the given field of cancer.
- In addition, ongoing changes in the trend where healthcare is moving towards patient-centered deliveries meant that the function of hospitals in oncology has become more prominent. With changes in the cancer treatments it is apparent that oncology is moving in the direction of personalized medicine whereby different treatments are applied to a particular individual depending on a set of factors that causes the cancer. This has been met by enhanced complex treatment regimens in hospitals that relies on genomic profiling and biomarkers to deliver percentage optimization on therapeutic interventions. Further, increased usage of outpatient care in the hospital infrastructure provides more versatile management approaches, from which patients can be treated at a shorter period of hospitalization. This change not only engages patients and increases their compliance and expected outcomes but also strengthens the place of hospitals as the key providers of quality cancer treatment.
Metastatic Castrate Resistant Prostate Cancer Treatment Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
- In North America, the decision of metastatic castrate-resistant prostate cancer (mCRPC) treatment is mainly influenced by highly developed healthcare system of North America that provides emphasis on new technologies and innovations. Many biotech and pharmaceutical companies in the United States advance in the development of drugs for oncology and among them, targeting prostate cancer. They expand clinical analysis capacities to assess new treatments; those useful next-generation androgen receptor inhibitors and immunotherapies which contribute to increasing patients’ survival rates and enhancing quality of life. FDA regulation is well developed, and rules promote the fast-track review of therapies with breakthrough potential, improving patients’ access to the treatment. Also, a wide web of academic research laboratories interface with industry stakeholders, creating the setting favourable for innovation and immediate transfer of research to the clinic.
- Also, a greater number of novel therapeutic options in development is a major driver of growth in the mCRPC market in North America due to higher patient incidence and earlier diagnosis. Through awareness campaigns, actions and enlightenment efforts, man well -being has upgraded hence more of them have come forward to do health check -ups especially early check of signs and approaches of prostate cancer. With increasing number of patients being diagnosed earlier and transitioning to MCRPC, the need for recent therapies in MCRPC also increases. In addition, there is growing use of teamwork when treating patients with prostate cancer using urologists, oncologists, and other support specialists that improve the care quality. The availability of brilliant treatment, a revolutionary approach of the North American healthcare system, and awareness levels of the patient make North America an essential place in the growth of mCRPC treatment market.
Active Key Players in the Metastatic Castrate Resistant Prostate Cancer Treatment Market
- F. Hoffmann-La Roche Ltd. (Switzerland),
- Teva Pharmaceutical Industries Ltd. (Ireland),
- Sanofi (France),
- Pfizer Inc. (U.S.),
- GSK plc (U.K.),
- Novartis AG (Switzerland),
- Bayer AG (Germany),
- Eli Lilly and Company (U.S.),
- Merck & Co., Inc. (U.S.),
- AstraZeneca (U.K.),
- Johnson & Johnson Private Limited (U.S.),
- Cipla (U.S.),
- Amneal Pharmaceuticals LLC. (U.S.),
- Bausch Health Companies Inc. (Canada),
- Takeda Pharmaceutical Company Limited (Japan),
- AbbVie Inc. (U.S.),
- Merck KGaA (Germany)
- Other Key Players
Key Industry Developments in the Metastatic Castrate Resistant Prostate Cancer Treatment Market:
- In March 2022, Novartis received FDA approval for Pluvicto (lutetium Lu 177 vipivotide tetraxetan), previously known as 177Lu-PSMA-617, for treating prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC). This innovative therapy marks the first FDA-approved targeted radioligand therapy (RLT), combining a ligand that targets PSMA with a therapeutic radioisotope. Pluvicto is designed to selectively deliver radiation to cancer cells while minimizing damage to healthy tissue
|
Global Metastatic Castrate Resistant Prostate Cancer Treatment Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 11.05 Bn. |
|
Forecast Period 2024-32 CAGR: |
8.7 % |
Market Size in 2032: |
USD 23.41 Bn. |
|
Segments Covered: |
By Treatment |
|
|
|
By Route of Administration |
|
||
|
By End-Users |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Metastatic Castrate Resistant Prostate Cancer Treatment Market by Treatment (2018-2032)
4.1 Metastatic Castrate Resistant Prostate Cancer Treatment Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Hormone Therapies
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Xofigo
4.5 Sipuleucel-T
4.6 Cabazitaxel
4.7 Docetaxel
4.8 Others
Chapter 5: Metastatic Castrate Resistant Prostate Cancer Treatment Market by Route of Administration (2018-2032)
5.1 Metastatic Castrate Resistant Prostate Cancer Treatment Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Oral
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Parenteral
5.5 Others
Chapter 6: Metastatic Castrate Resistant Prostate Cancer Treatment Market by End-Users (2018-2032)
6.1 Metastatic Castrate Resistant Prostate Cancer Treatment Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Hospitals
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Specialty Clinics
6.5 Homecare
6.6 Others
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Metastatic Castrate Resistant Prostate Cancer Treatment Market Share by Manufacturer (2024)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 F. HOFFMANN-LA ROCHE LTD. (SWITZERLAND) TEVA PHARMACEUTICAL INDUSTRIES LTD. (IRELAND) SANOFI (FRANCE) PFIZER INC. (U.S.) GSK PLC (U.K.) NOVARTIS AG (SWITZERLAND) BAYER AG (GERMANY) ELI LILLY AND COMPANY (U.S.) MERCK & COINC. (U.S.) ASTRAZENECA (U.K.) JOHNSON & JOHNSON PRIVATE LIMITED (U.S.) CIPLA (U.S.) AMNEAL PHARMACEUTICALS LLC. (U.S.) BAUSCH HEALTH COMPANIES INC. (CANADA) TAKEDA PHARMACEUTICAL COMPANY LIMITED (JAPAN) ABBVIE INC. (U.S.) MERCK KGAA (GERMANY)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 OTHER KEY PLAYERS
Chapter 8: Global Metastatic Castrate Resistant Prostate Cancer Treatment Market By Region
8.1 Overview
8.2. North America Metastatic Castrate Resistant Prostate Cancer Treatment Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size by Treatment
8.2.4.1 Hormone Therapies
8.2.4.2 Xofigo
8.2.4.3 Sipuleucel-T
8.2.4.4 Cabazitaxel
8.2.4.5 Docetaxel
8.2.4.6 Others
8.2.5 Historic and Forecasted Market Size by Route of Administration
8.2.5.1 Oral
8.2.5.2 Parenteral
8.2.5.3 Others
8.2.6 Historic and Forecasted Market Size by End-Users
8.2.6.1 Hospitals
8.2.6.2 Specialty Clinics
8.2.6.3 Homecare
8.2.6.4 Others
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Metastatic Castrate Resistant Prostate Cancer Treatment Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size by Treatment
8.3.4.1 Hormone Therapies
8.3.4.2 Xofigo
8.3.4.3 Sipuleucel-T
8.3.4.4 Cabazitaxel
8.3.4.5 Docetaxel
8.3.4.6 Others
8.3.5 Historic and Forecasted Market Size by Route of Administration
8.3.5.1 Oral
8.3.5.2 Parenteral
8.3.5.3 Others
8.3.6 Historic and Forecasted Market Size by End-Users
8.3.6.1 Hospitals
8.3.6.2 Specialty Clinics
8.3.6.3 Homecare
8.3.6.4 Others
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Metastatic Castrate Resistant Prostate Cancer Treatment Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size by Treatment
8.4.4.1 Hormone Therapies
8.4.4.2 Xofigo
8.4.4.3 Sipuleucel-T
8.4.4.4 Cabazitaxel
8.4.4.5 Docetaxel
8.4.4.6 Others
8.4.5 Historic and Forecasted Market Size by Route of Administration
8.4.5.1 Oral
8.4.5.2 Parenteral
8.4.5.3 Others
8.4.6 Historic and Forecasted Market Size by End-Users
8.4.6.1 Hospitals
8.4.6.2 Specialty Clinics
8.4.6.3 Homecare
8.4.6.4 Others
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Metastatic Castrate Resistant Prostate Cancer Treatment Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size by Treatment
8.5.4.1 Hormone Therapies
8.5.4.2 Xofigo
8.5.4.3 Sipuleucel-T
8.5.4.4 Cabazitaxel
8.5.4.5 Docetaxel
8.5.4.6 Others
8.5.5 Historic and Forecasted Market Size by Route of Administration
8.5.5.1 Oral
8.5.5.2 Parenteral
8.5.5.3 Others
8.5.6 Historic and Forecasted Market Size by End-Users
8.5.6.1 Hospitals
8.5.6.2 Specialty Clinics
8.5.6.3 Homecare
8.5.6.4 Others
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Metastatic Castrate Resistant Prostate Cancer Treatment Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size by Treatment
8.6.4.1 Hormone Therapies
8.6.4.2 Xofigo
8.6.4.3 Sipuleucel-T
8.6.4.4 Cabazitaxel
8.6.4.5 Docetaxel
8.6.4.6 Others
8.6.5 Historic and Forecasted Market Size by Route of Administration
8.6.5.1 Oral
8.6.5.2 Parenteral
8.6.5.3 Others
8.6.6 Historic and Forecasted Market Size by End-Users
8.6.6.1 Hospitals
8.6.6.2 Specialty Clinics
8.6.6.3 Homecare
8.6.6.4 Others
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Metastatic Castrate Resistant Prostate Cancer Treatment Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size by Treatment
8.7.4.1 Hormone Therapies
8.7.4.2 Xofigo
8.7.4.3 Sipuleucel-T
8.7.4.4 Cabazitaxel
8.7.4.5 Docetaxel
8.7.4.6 Others
8.7.5 Historic and Forecasted Market Size by Route of Administration
8.7.5.1 Oral
8.7.5.2 Parenteral
8.7.5.3 Others
8.7.6 Historic and Forecasted Market Size by End-Users
8.7.6.1 Hospitals
8.7.6.2 Specialty Clinics
8.7.6.3 Homecare
8.7.6.4 Others
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
|
Global Metastatic Castrate Resistant Prostate Cancer Treatment Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 11.05 Bn. |
|
Forecast Period 2024-32 CAGR: |
8.7 % |
Market Size in 2032: |
USD 23.41 Bn. |
|
Segments Covered: |
By Treatment |
|
|
|
By Route of Administration |
|
||
|
By End-Users |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













