Global Clinical Trial Outsourcing Market Synopsis
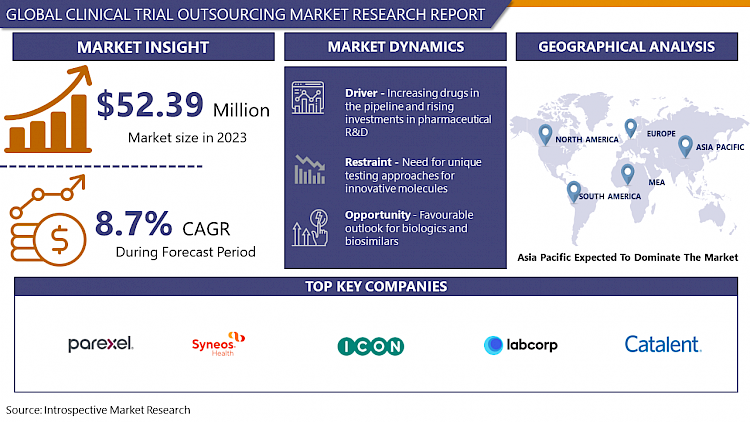
Global Clinical Trial Outsourcing Market Size Was Valued at USD 52.39 Billion in 2023, and is Projected to Reach USD 111.01 Billion by 2032, Growing at a CAGR of 8.7% From 2024-2032.
- Clinical trial outsourcing refers to the practice where pharmaceutical, biotechnology, or medical device companies collaborate with Contract Research Organizations (CROs) or other specialized service providers to delegate various aspects of the clinical trial process.
- The clinical trial outsourcing market has experienced significant growth and evolution in recent years due to various factors reshaping the pharmaceutical and biotechnology industries. Outsourcing clinical trials has become increasingly prevalent as companies seek to streamline operations, reduce costs, and access specialized expertise. This market's growth is propelled by the rising complexity of clinical trials, advancements in technology, and the global expansion of research and development activities.
- The market is witnessing a shift toward strategic partnerships and collaborations between pharmaceutical companies and Contract Research Organizations (CROs). These partnerships allow for efficient resource utilization, increased flexibility, and access to a broader range of services and expertise. Moreover, the demand for specialized services such as clinical data management, regulatory affairs, and biostatistics has fuelled the growth of niche CROs catering to specific therapeutic areas or phases of clinical trials.
Clinical Trial Outsourcing Market Trend Analysis:
Increasing Drugs in The Pipeline and Rising Investments in Pharmaceutical R&D
- The pharmaceutical industry has experienced a surge in drug development initiatives and a substantial uptick in investments dedicated to research and development (R&D). This growth can be attributed to several factors, including advancements in scientific understanding, technological innovations, and an evolving regulatory landscape. One significant driver is the pressing need to address unmet medical needs and combat complex diseases that continue to pose significant challenges globally.
- The emergence of ground-breaking technologies, such as genomics, precision medicine, and biotechnology, has accelerated the drug discovery process. This has led to an expansion in the number of potential drug candidates in the pipeline. The ability to delve deeper into the genetic and molecular basis of diseases has opened new avenues for developing targeted therapies, leading to a heightened enthusiasm for R&D investments across the pharmaceutical sector.
- Parallelly, the convergence of diverse disciplines, including artificial intelligence (AI), machine learning, and big data analytics, has revolutionized the way researchers explore and identify potential drug targets. These tools enable efficient screening of compounds, predictive modeling of drug interactions, and optimization of clinical trial designs, significantly reducing the time and resources required for drug development.
Favorable Outlook for Biologics and Biosimilars
- The burgeoning market for biologics and biosimilars presents a significant opportunity within the realm of clinical trial outsourcing. Biologics, derived from living organisms, offer innovative therapeutic options for various complex diseases, driving a surge in their development. Simultaneously, the increasing interest in biosimilars, which are highly similar versions of approved biologic products, presents a cost-effective alternative and fosters competition in the market.
- The complex nature of biologics demands specialized expertise and rigorous testing, creating a robust demand for outsourced clinical trials. Contract Research Organizations (CROs) equipped with expertise in handling the intricacies of biological compounds are increasingly sought after by pharmaceutical companies aiming to navigate the complexities of these trials effectively. From design and manufacturing to clinical testing and regulatory compliance, outsourcing allows companies to tap into specialized knowledge and resources required for successful biologic development.
Clinical Trial Outsourcing Market Segment Analysis:
Clinical Trial Outsourcing Market Segmented on the basis of phase, service type, therapeutic area, and application.
By Phase, Phase III segment is expected to dominate the market during the forecast period
The dominance of the Phase III segment within the clinical trial outsourcing market during the forecast period signifies a critical stage in drug development where outsourced trials play a pivotal role. Phase III trials are the culmination of extensive preclinical and Phase I/II studies, focusing on assessing the efficacy and safety of a drug candidate in a larger patient population. This phase often involves a substantial number of participants across multiple sites, demanding extensive resources, expertise, and efficient management, which drives the reliance on outsourcing.
The complexity and scale of Phase III trials necessitate collaboration with Contract Research Organizations (CROs) possessing a wide array of capabilities, including patient recruitment strategies, diverse geographic reach, regulatory expertise, and data management proficiency.
By Service Type, Analytical Testing Services segment held the largest share of 31.6% in 2022
- Analytical testing services encompass a wide spectrum of activities, including chemical, physical, and biological analyses of drug compounds, formulations, and final products. This segment's significance lies in its contribution to meeting stringent regulatory standards and confirming the integrity and consistency of therapeutic agents.
- Pharmaceutical companies increasingly rely on outsourcing analytical testing services to specialized Contract Research Organizations (CROs) equipped with state-of-the-art laboratories and expertise in various analytical techniques
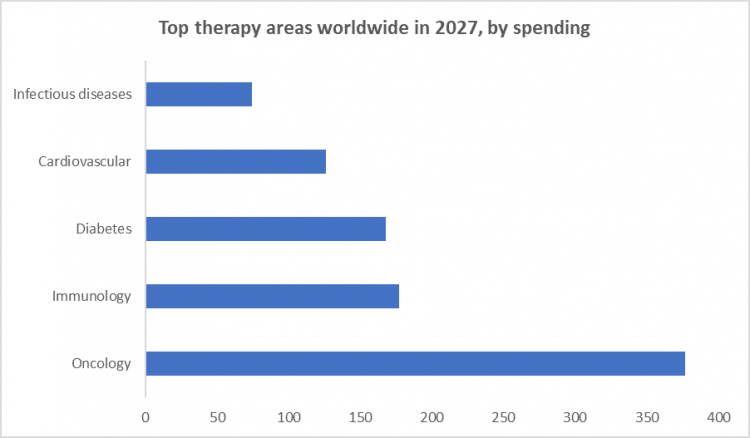
By Therapeutic Area, Oncology segment held the largest share of 35% in 2022
- Oncology stands as a leading therapeutic area due to the complex nature of cancer and the continuous quest for more effective treatments. The high prevalence of various cancer types globally has propelled pharmaceutical companies to intensify their focus on developing innovative oncology drugs, fostering a strong demand for outsourced clinical trials.
- Therapies are tailored to specific genetic profiles or molecular characteristics of tumors, adding another layer of complexity to clinical trials. This trend has further fuelled the reliance on outsourcing, as specialized CROs possess the expertise to design and execute trials
By Application, Monoclonal Antibodies segment held the largest share of 29% in 2022
- Monoclonal antibodies, engineered to target specific antigens, have gained immense traction in treating conditions such as cancer, autoimmune disorders, and infectious diseases. This prominence in clinical trial outsourcing reflects the industry's emphasis on developing and refining mAbs as a cornerstone of modern therapeutic interventions.
- The complex development process and diverse applications of monoclonal antibodies necessitate specialized expertise, driving pharmaceutical companies to outsource these trials to Contract Research Organizations (CROs) equipped with advanced capabilities in biologics development.
Clinical Trial Outsourcing Market Regional Insights:
Asia Pacific is Expected to Dominate the Market Over the Forecast period
The United States, in particular, leads in clinical research and outsourcing activities, attracting a significant portion of global clinical trials across various therapeutic areas. It boasts a wealth of expertise in advanced technologies, a large pool of skilled professionals, and a conducive environment for conducting trials efficiently.
Clinical Trial Outsourcing Market Top Key Players:
- PAREXEL (US)
- IQVIA (US)
- SYNEOS HEALTH (US)
- ICON PLC(US)
- MEDIDATA SOLUTIONS (US)
- PRA HEALTH SCIENCES (US)
- COVANCE (US)
- CHARLES RIVER LABORATORIES (US)
- CATALENT (US)
- EMMES COMPANY (US)
- SYNEOS HEALTH (US)
- FORTREA INC. (US)
- ADVANCED CLINICAL (US)
- THERMO FISHER SCIENTIFIC INC. (US)
- FRONTAGE LABS (US)
- ACM GLOBAL LABORATORIES (US)
- WORLDWIDE CLINICAL TRIALS (US)
- CTI CLINICAL TRIAL & CONSULTING (US)
- FIRMA CLINICAL RESEARCH (US)
- CELERION (US)
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH (EUROPE)
- PHARMASERV INTERNATIONAL (GERMANY)
- APTIV SOLUTIONS (FRANCE)
- DOVE QUALITY SOLUTIONS (UK)
- CLINIGEN GROUP (UK)
Key Industry Developments in the Clinical Trial Outsourcing Market:
In March 2023, Syneos Health entered into a multiyear agreement with Microsoft to create a platform that uses machine learning to elevate biopharma companies’ commercial performance and speed up clinical trial analysis, planning, and operation.
In September 2022, Parexel International established a new clinical trial supplies and logistics facility in Suzhou, China. This facility provides both local and international biopharmaceutical clients with quick access to clinical trial materials and medications for sites and patients, thus expediting the progress of clinical trials in the region.
|
Clinical Trial Outsourcing Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 52.39 Bn. |
|
CAGR (2023-2030) : |
8.7% |
Market Size in 2032: |
USD 111.01 Bn. |
|
Segments Covered: |
By Phase |
|
|
|
By Service Type |
|
||
|
By Therapeutic Area |
|
||
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the Report: |
|
||
- INTRODUCTION
- RESEARCH OBJECTIVES
- RESEARCH METHODOLOGY
- RESEARCH PROCESS
- SCOPE AND COVERAGE
- Market Definition
- Key Questions Answered
- MARKET SEGMENTATION
- EXECUTIVE SUMMARY
- MARKET OVERVIEW
- GROWTH OPPORTUNITIES BY SEGMENT
- MARKET LANDSCAPE
- PORTER’S FIVE FORCES ANALYSIS
- Bargaining Power Of Supplier
- Threat Of New Entrants
- Threat Of Substitutes
- Competitive Rivalry
- Bargaining Power Among Buyers
- INDUSTRY VALUE CHAIN ANALYSIS
- MARKET DYNAMICS
- Drivers
- Restraints
- Opportunities
- Challenges
- MARKET TREND ANALYSIS
- REGULATORY LANDSCAPE
- PESTLE ANALYSIS
- PRICE TREND ANALYSIS
- PATENT ANALYSIS
- TECHNOLOGY EVALUATION
- MARKET IMPACT OF THE RUSSIA-UKRAINE WAR
- Geopolitical Market Disruptions
- Supply Chain Disruptions
- Instability in Emerging Markets
- ECOSYSTEM
- PORTER’S FIVE FORCES ANALYSIS
- Clinical Trial Outsourcing MARKET BY PHASE (2017-2032)
- Clinical Trial Outsourcing MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- PHASE I
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- PHASE II
- PHASE III
- PHASE IV
- Clinical Trial Outsourcing MARKET BY SERVICE TYPE (2017-2032)
- Clinical Trial Outsourcing MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- LABORATORY SERVICES
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- BIOANALYTICAL TESTING SERVICES
- DECENTRALIZED CLINICAL TRIAL SERVICES
- ANALYTICAL TESTING SERVICES
- Clinical Trial Outsourcing MARKET BY THERAPEUTIC AREA (2017-2032)
- Clinical Trial Outsourcing MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- ONCOLOGY
- Introduction and Market Overview
- Historic and Forecasted Market Size in Value (2017 – 2032F)
- Historic and Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors and Opportunities
- Geographic Segmentation Analysis
- INFECTIOUS DISEASES
- NEUROLOGY
- METABOLIC
- DISORDERS
- IMMUNOLOGY
- Clinical Trial Outsourcing MARKET BY APPLICATION (2017-2032)
- Clinical Trial Outsourcing MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- SMALL MOLECULES
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- MONOCLONAL ANTIBODIES
- VACCINE
- CELL & GENE THERAPY
- COMPANY PROFILES AND COMPETITIVE ANALYSIS
- COMPETITIVE LANDSCAPE
- Competitive Positioning
- Clinical Trial Outsourcing Market Share By Manufacturer (2023)
- Industry BCG Matrix
- Heat Map Analysis
- Mergers & Acquisitions
- PAREXEL
- Company Overview
- Key Executives
- Company Snapshot
- Role of the Company in the Market
- Sustainability and Social Responsibility
- Operating Business Segments
- Product Portfolio
- Business Performance (Production Volume, Sales Volume, Sales Margin, Production Capacity, Capacity Utilization Rate)
- Key Strategic Moves And Recent Developments
- SWOT Analysis
- IQVIA (US)
- SYNEOS HEALTH (US)
- ICON PLC (US)
- MEDIDATA SOLUTIONS (US)
- PRA HEALTH SCIENCES (US)
- COVANCE (US)
- CHARLES RIVER LABORATORIES (US)
- CATALENT (US)
- EMMES COMPANY (US)
- SYNEOS HEALTH (US)
- FORTREA INC. (US)
- ADVANCED CLINICAL (US)
- THERMO FISHER SCIENTIFIC INC. (US)
- FRONTAGE LABS (US)
- ACM GLOBAL LABORATORIES (US)
- WORLDWIDE CLINICAL TRIALS (US)
- CTI CLINICAL TRIAL & CONSULTING (US)
- FIRMA CLINICAL RESEARCH (US)
- CELERION (US)
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH (EUROPE)
- PHARMASERV INTERNATIONAL (GERMANY)
- APTIV SOLUTIONS (FRANCE)
- DOVE QUALITY SOLUTIONS (UK)
- CLINIGEN GROUP (UK)
- COMPETITIVE LANDSCAPE
- GLOBAL Clinical Trial Outsourcing MARKET BY REGION
- OVERVIEW
- NORTH AMERICA
- Key Market Trends, Growth Factors and Opportunities
- Key Manufacturers
- Historic and Forecasted Market Size by Phase
- Historic And Forecasted Market Size By Service Type
- Historic And Forecasted Market Size By Therapeutic Area
- Historic And Forecasted Market Size By Application
- Historic And Forecasted Market Size By Country
- USA
- Canada
- Mexico
- EASTERN EUROPE
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Russia
- Bulgaria
- The Czech Republic
- Hungary
- Poland
- Romania
- Rest Of Eastern Europe
- WESTERN EUROPE
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Germany
- United Kingdom
- France
- The Netherlands
- Italy
- Spain
- Rest Of Western Europe
- ASIA PACIFIC
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Vietnam
- The Philippines
- Australia
- New-Zealand
- Rest Of APAC
- MIDDLE EAST & AFRICA
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Turkey
- Bahrain
- Kuwait
- Saudi Arabia
- Qatar
- UAE
- Israel
- South Africa
- SOUTH AMERICA
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Brazil
- Argentina
- Rest of South America
- INVESTMENT ANALYSIS
- ANALYST VIEWPOINT AND CONCLUSION
- Recommendations and Concluding Analysis
- Potential Market Strategies
|
Clinical Trial Outsourcing Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 52.39 Bn. |
|
CAGR (2023-2030) : |
8.7% |
Market Size in 2032: |
USD 111.01 Bn. |
|
Segments Covered: |
By Phase |
|
|
|
By Service Type |
|
||
|
By Therapeutic Area |
|
||
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the Report: |
|
||
Frequently Asked Questions :
The forecast period in the Clinical Trial Outsourcing Market research report is 2024-2032.
Parexel (US), IQVIA (US), Syneos Health (US), ICON plc (US), Medidata Solutions (US), PRA Health Sciences (US), Covance (US), Charles River Laboratories (US), Catalent (US), Emmes Company (US), Syneos Health (US), Fortrea Inc. (US), Advanced Clinical (US), Thermo Fisher Scientific Inc. (US), Frontage Labs (US), ACM Global Laboratories (US), Worldwide Clinical Trials (US), CTI Clinical Trial & Consulting (US), Firma Clinical Research (US), Celerion (US), Boehringer Ingelheim International GmbH (Europe), Pharmaserv International (Germany), Aptiv Solutions (France), Dove Quality Solutions (UK), Clinigen Group (UK), and Other Major Players.
The Clinical Trial Outsourcing Market is segmented into Phase, Service Type, Therapeutic Area, Application, and region. By Phase, the market is categorized into Phase I, Phase II, Phase III, and Phase IV. By Service Type, the market is categorized into Laboratory Services, Bioanalytical Testing Services, Decentralized Clinical Trial Services, and Analytical Testing Services. By Therapeutic Area, the market is categorized into Oncology, Infectious Diseases, Neurology, Metabolic, Disorders, and Immunology. By region, it is analyzed across North America (U.S.; Canada; Mexico), Eastern Europe (Bulgaria; The Czech Republic; Hungary; Poland; Romania; Rest of Eastern Europe), Western Europe (Germany; UK; France; Netherlands; Italy; Russia; Spain; Rest of Western Europe), Asia-Pacific (China; India; Japan; Southeast Asia, etc.), South America (Brazil; Argentina, etc.), Middle East & Africa (Saudi Arabia; South Africa, etc.).
Clinical trial outsourcing refers to the practice where pharmaceutical, biotechnology, or medical device companies collaborate with Contract Research Organizations (CROs) or other specialized service providers to delegate various aspects of the clinical trial process.
Global Clinical Trial Outsourcing Market Size Was Valued at USD 52.39 Billion in 2023, and is Projected to Reach USD 111.01 Billion by 2032, Growing at a CAGR of 8.7% From 2024-2032.


































