Global Clinical Trial Management Software Market Overview
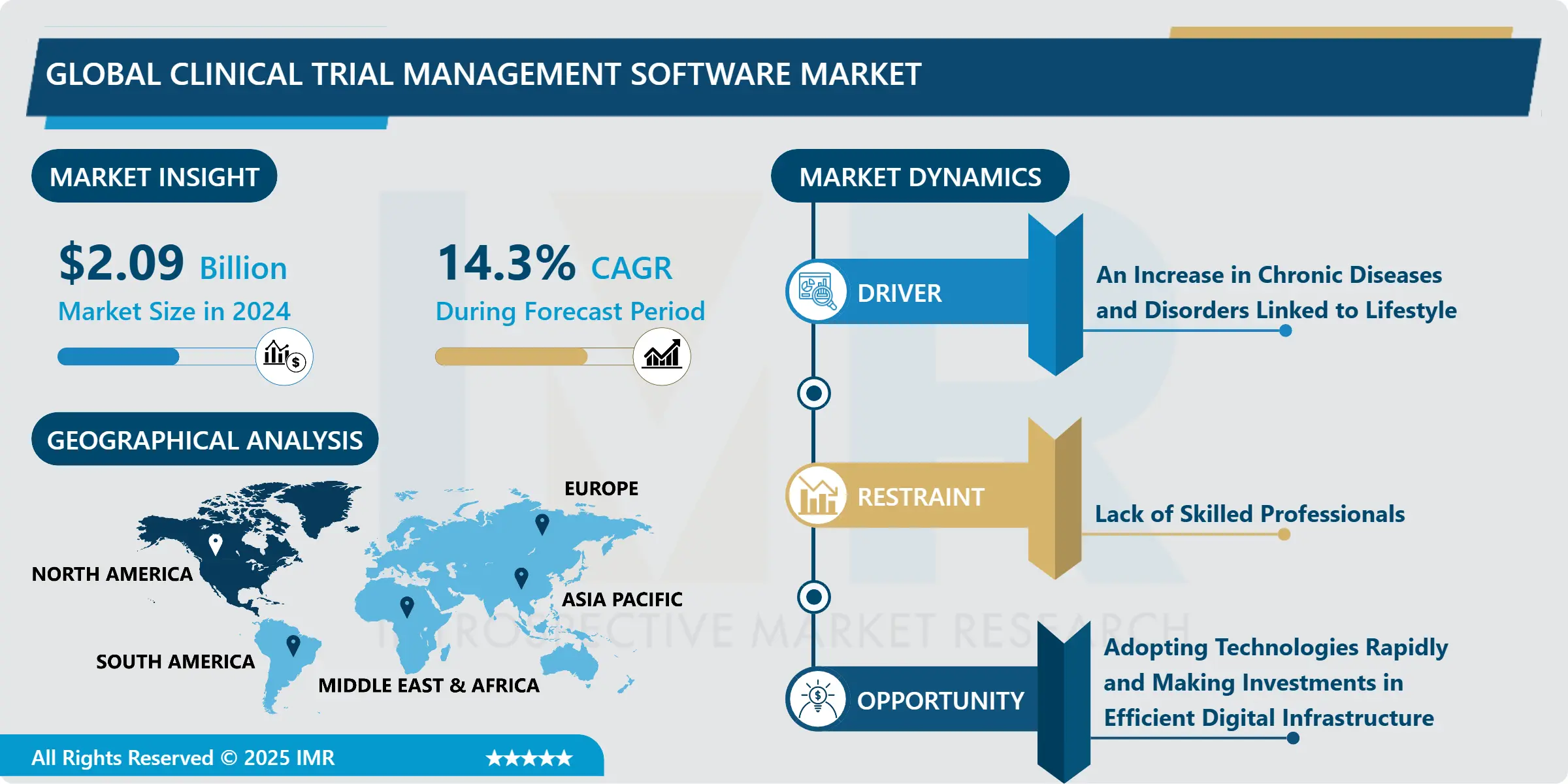
Global Clinical Trial Management Software Market size is expected to grow from USD 2.09 Billion in 2024 to USD 6.09 Billion by 2032, at a CAGR of 14.3% during the forecast period 2025-2032.
Global Clinical Trial Management Software (CTMS) is a specialized software designed to help manage clinical trials efficiently. Clinical trials are essential for testing the safety and efficacy of new drugs, medical devices, and treatments before they can be approved for public use. Managing these trials involves complex tasks, data collection, regulatory compliance, and collaboration among multiple stakeholders, including researchers, sponsors, regulatory agencies, and participants. The trial planning and design process involves defining protocols and study parameters. Patient recruitment and enrollment are also crucial. Real-time data collection and monitoring are facilitated. Regulatory compliance is ensured, maintaining ethical and legal guidelines. Financial management involves managing budgeting, transactions, and invoicing. Communication and collaboration are enhanced among team members, investigators, and sponsors. Reporting and analysis provide a comprehensive understanding of the trial's progress and outcomes.
The use of automated data entry systems in clinical trials offers numerous benefits, including time savings, enhanced data accuracy, improved regulatory compliance, better resource management, streamlined communication, centralized data access, and proactive risk management. These systems automate manual processes, reduce errors in data entry, and ensure ethical and legal conduct of clinical trials. They also facilitate the efficient allocation of resources, promote data transparency, and enable proactive risk management, ensuring a more coordinated and effective research environment.
Clinical Trial Management Software Market Trend Analysis:
An Increase in Chronic Diseases and Disorders Linked to Lifestyle
-
The market for clinical trial management software, or CTMS, is growing as a result of the rise in the frequency of chronic illnesses and disorders associated with lifestyle choices. Diseases like diabetes, heart disease, and some types of cancer are becoming more common. These conditions are linked to things like poor eating habits, stress, and sedentary lives. There has been a discernible surge in clinical studies designed to address these chronic lifestyle-related illnesses with novel therapies, medications, and interventions in response to this global health crisis.
-
The increasing usage of Clinical Trial Management Software can be attributed to the necessity for effective management of these complex clinical studies. These software programs are essential for simplifying the many procedures that go into organizing, carrying out, and overseeing these experiments. Improved data accuracy, regulatory compliance, and improved research team cooperation are all made possible by CTMS and are essential to the effective conduct of clinical trials.
-
The market for clinical trial management software is expected to grow steadily as a result of the healthcare sector focusing its efforts on creating cutting-edge treatments to counter the rising prevalence of chronic diseases linked to poor lifestyle choices. This software offers the infrastructure and necessary tools to support the changing face of clinical research.
Adopting Technologies Rapidly and Making Investments in Efficient Digital Infrastructure
-
The potential of digital technology to revolutionize and expedite clinical trial processes is being recognized by the healthcare sector on a growing scale. The incorporation of sophisticated Clinical Trial Management Software (CTMS) solutions becomes imperative as businesses strive to augment their research capacities and optimize their operational efficacy.
-
Research teams may leverage data analytics, automation, and artificial intelligence to administer clinical trials more efficiently when they adopt technology quickly. CTMS digital solutions, which handle everything from patient recruiting and data collecting to guaranteeing regulatory compliance and real-time monitoring, help to improve overall trial productivity.
-
Moreover, investments in robust digital infrastructure are essential for guaranteeing data security, uninterrupted connectivity, and productive cooperation between geographically separated research teams and stakeholders. The clinical research ecosystem is benefiting from the adoption of digital transformation, which not only speeds up trial schedules and helps with logistical issues, but also improves the quality of data gathered, which is driving the CTMS market.
Clinical Trial Management Software Market Segment Analysis:
Clinical Trial Management Software Market is Segmented Based on By Deployment, Delivery, Component and End-User.
By End-User, Large Pharma-Biotech Companies Segment Is Expected to Dominate the Market During the Forecast Period.
-
The Pharmaceutical and Biotechnology Firms sector is expected to hold a dominant market share in the Clinical Trial Management Software (CTMS) market due to its stable and steady growth trajectory. These sectors are driving the need for effective and all-encompassing clinical trial management systems by actively participating in cutting-edge research and development projects. The benefits that CTMS offers in terms of expediting trial procedures, guaranteeing regulatory compliance, and encouraging research team cooperation are being recognized by pharmaceutical and biotechnology businesses on a growing basis. Fast-tracking medication development and improving overall research outcomes are made possible by CTMS's essential requirements for smooth data management, real-time monitoring, and adherence to strict industry standards. Pharmaceutical and biotechnology companies are driving the significant adoption and market dominance of CTMS with a sharp focus on innovation and the growing complexity of clinical trials.
By Deployment, the Enterprise-wide CTMS Segment Held the Largest Share In 2024
-
The category for enterprise-wide clinical trial management software (CTMS) has the most market share. This dominance is a consequence of enterprises looking for effective administration and supervision of their whole clinical trial portfolio widely implementing full CTMS systems. Study design, participant recruiting, data administration, regulatory compliance, and financial tracking are just a few of the functions that enterprise-wide CTMS offers as a single, integrated platform. This comprehensive strategy guarantees smooth departmental coordination throughout the clinical trial procedure while also greatly improving operational efficiency.
-
The requirement for a centralized system that can handle the complexities of handling several trials at once is the reason enterprise-wide solutions are preferred. The significance of this sector demonstrates how the industry recognizes the value that integrated CTMS systems bring in terms of improving data accuracy, expediting procedures, and ensuring regulatory compliance throughout the whole range of clinical research operations.
Clinical Trial Management Software Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
-
The sophisticated healthcare infrastructure, significant R&D activities, and large concentration of pharmaceutical and biotechnology companies in North America are the main due to behind the region's projected leadership in the worldwide Clinical Trial Management System (CTMS) market. An effective regulatory structure and a proactive approach to implementing cutting-edge technology support the region's leadership even more. The established clinical research ecosystem in North America, which includes prominent research institutes and clinical trial sites, makes the region a key participant in the CTMS industry. North America serves as a primary center for the adoption and growth of CTMS technologies due to its leadership in improving medical research and its hospitable regulatory environment.
Key Players Covered in Clinical Trial Management Software Market:
- Merge Healthcare Incorporated (U.S.)
- Bio-Optronics (U.S.)
- DSG INC (U.S.)
- ERT Clinical Bioclinica (U.S.)
- Oracle Corporation (U.S.)
- Medidata Solutions (U.S.)
- DATATRAK International, Inc. (U.S.)
- MedNet Solutions, Inc., (U.S.)
- Parexel International (U.S.)
- IBM (U.S.)
- ArisGlobal (U.S.)
- Veeva Systems (U.S.)
- MasterControl (U.S.)
- DZS Software Solutions (U.S.)
- RealTime Software Solutions LLC (U.S.)
- Advarra Technology Solutions (U.S.)
- Calyx (U.S.)
- Crucial Data Solutions (U.S.)
- ICON plc (Ireland)
- Ennov (France)
- Other Active Players
Key Industry Development in The Clinical Trial Management Software Market:
-
In February 2024, Revvity Signals, the software and informatics arm of Revvity, announced the launch of Signals Clinical, a groundbreaking software-as-a-service (SaaS) platform. This end-to-end solution centralizes clinical trial data, providing rapid, actionable insights for expedited decision-making. Integrated with enterprise-class Spotfire visual analytics, Signals Clinical revolutionizes data handling in pharmaceutical, biotech, and contract research sectors. This platform accelerates clinical development and therapeutic innovation, ensuring faster delivery of new therapeutics to market. Experience the future of clinical data science with Signals Clinicals.
-
In March 2023, Assentia launched tech platforms to support payments in the clinical trial space. The company released two SaaS-based applications, GrantPay and GrantPact, to provide clinical trial contract negotiation and payment services.
|
Global Clinical Trial Management Software Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2032 |
|
Historical Data: |
2018 to 2023 |
Market Size in 2024: |
USD 2.09 Bn. |
|
Forecast Period 2024-32 CAGR: |
14.3% |
Market Size in 2032: |
USD 6.09 Bn. |
|
Segments Covered: |
By Deployment |
|
|
|
By Delivery |
|
||
|
By Component |
|
||
|
By End-User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Clinical Trial Management Software Market by Deployment (2018-2032)
4.1 Clinical Trial Management Software Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Enterprise-wide CTMS
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 On-Site CTMS
Chapter 5: Clinical Trial Management Software Market by Delivery (2018-2032)
5.1 Clinical Trial Management Software Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Web-based
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Licensed Enterprise
5.5 Cloud-based
Chapter 6: Clinical Trial Management Software Market by Component (2018-2032)
6.1 Clinical Trial Management Software Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Software
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Services
Chapter 7: Clinical Trial Management Software Market by End-User (2018-2032)
7.1 Clinical Trial Management Software Market Snapshot and Growth Engine
7.2 Market Overview
7.3 Pharmaceutical and Biotechnology Firms
7.3.1 Introduction and Market Overview
7.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
7.3.3 Key Market Trends, Growth Factors, and Opportunities
7.3.4 Geographic Segmentation Analysis
7.4 CROs
7.5 Medical Device Firms
Chapter 8: Company Profiles and Competitive Analysis
8.1 Competitive Landscape
8.1.1 Competitive Benchmarking
8.1.2 Clinical Trial Management Software Market Share by Manufacturer (2024)
8.1.3 Industry BCG Matrix
8.1.4 Heat Map Analysis
8.1.5 Mergers and Acquisitions
8.2 ORACLE CORPORATION
8.2.1 Company Overview
8.2.2 Key Executives
8.2.3 Company Snapshot
8.2.4 Role of the Company in the Market
8.2.5 Sustainability and Social Responsibility
8.2.6 Operating Business Segments
8.2.7 Product Portfolio
8.2.8 Business Performance
8.2.9 Key Strategic Moves and Recent Developments
8.2.10 SWOT Analysis
8.3 MICROSOFT CORPORATION
8.4 IBM CORPORATION
8.5 AMAZON WEB SERVICES
8.6 GOOGLE INCCISCO SYSTEMS
8.7 RIVERMEADOW SOFTWARE
8.8 RACKSPACE US
8.9 INFORMATICA
8.10 OVH US LLC
Chapter 9: Global Clinical Trial Management Software Market By Region
9.1 Overview
9.2. North America Clinical Trial Management Software Market
9.2.1 Key Market Trends, Growth Factors and Opportunities
9.2.2 Top Key Companies
9.2.3 Historic and Forecasted Market Size by Segments
9.2.4 Historic and Forecasted Market Size by Deployment
9.2.4.1 Enterprise-wide CTMS
9.2.4.2 On-Site CTMS
9.2.5 Historic and Forecasted Market Size by Delivery
9.2.5.1 Web-based
9.2.5.2 Licensed Enterprise
9.2.5.3 Cloud-based
9.2.6 Historic and Forecasted Market Size by Component
9.2.6.1 Software
9.2.6.2 Services
9.2.7 Historic and Forecasted Market Size by End-User
9.2.7.1 Pharmaceutical and Biotechnology Firms
9.2.7.2 CROs
9.2.7.3 Medical Device Firms
9.2.8 Historic and Forecast Market Size by Country
9.2.8.1 US
9.2.8.2 Canada
9.2.8.3 Mexico
9.3. Eastern Europe Clinical Trial Management Software Market
9.3.1 Key Market Trends, Growth Factors and Opportunities
9.3.2 Top Key Companies
9.3.3 Historic and Forecasted Market Size by Segments
9.3.4 Historic and Forecasted Market Size by Deployment
9.3.4.1 Enterprise-wide CTMS
9.3.4.2 On-Site CTMS
9.3.5 Historic and Forecasted Market Size by Delivery
9.3.5.1 Web-based
9.3.5.2 Licensed Enterprise
9.3.5.3 Cloud-based
9.3.6 Historic and Forecasted Market Size by Component
9.3.6.1 Software
9.3.6.2 Services
9.3.7 Historic and Forecasted Market Size by End-User
9.3.7.1 Pharmaceutical and Biotechnology Firms
9.3.7.2 CROs
9.3.7.3 Medical Device Firms
9.3.8 Historic and Forecast Market Size by Country
9.3.8.1 Russia
9.3.8.2 Bulgaria
9.3.8.3 The Czech Republic
9.3.8.4 Hungary
9.3.8.5 Poland
9.3.8.6 Romania
9.3.8.7 Rest of Eastern Europe
9.4. Western Europe Clinical Trial Management Software Market
9.4.1 Key Market Trends, Growth Factors and Opportunities
9.4.2 Top Key Companies
9.4.3 Historic and Forecasted Market Size by Segments
9.4.4 Historic and Forecasted Market Size by Deployment
9.4.4.1 Enterprise-wide CTMS
9.4.4.2 On-Site CTMS
9.4.5 Historic and Forecasted Market Size by Delivery
9.4.5.1 Web-based
9.4.5.2 Licensed Enterprise
9.4.5.3 Cloud-based
9.4.6 Historic and Forecasted Market Size by Component
9.4.6.1 Software
9.4.6.2 Services
9.4.7 Historic and Forecasted Market Size by End-User
9.4.7.1 Pharmaceutical and Biotechnology Firms
9.4.7.2 CROs
9.4.7.3 Medical Device Firms
9.4.8 Historic and Forecast Market Size by Country
9.4.8.1 Germany
9.4.8.2 UK
9.4.8.3 France
9.4.8.4 The Netherlands
9.4.8.5 Italy
9.4.8.6 Spain
9.4.8.7 Rest of Western Europe
9.5. Asia Pacific Clinical Trial Management Software Market
9.5.1 Key Market Trends, Growth Factors and Opportunities
9.5.2 Top Key Companies
9.5.3 Historic and Forecasted Market Size by Segments
9.5.4 Historic and Forecasted Market Size by Deployment
9.5.4.1 Enterprise-wide CTMS
9.5.4.2 On-Site CTMS
9.5.5 Historic and Forecasted Market Size by Delivery
9.5.5.1 Web-based
9.5.5.2 Licensed Enterprise
9.5.5.3 Cloud-based
9.5.6 Historic and Forecasted Market Size by Component
9.5.6.1 Software
9.5.6.2 Services
9.5.7 Historic and Forecasted Market Size by End-User
9.5.7.1 Pharmaceutical and Biotechnology Firms
9.5.7.2 CROs
9.5.7.3 Medical Device Firms
9.5.8 Historic and Forecast Market Size by Country
9.5.8.1 China
9.5.8.2 India
9.5.8.3 Japan
9.5.8.4 South Korea
9.5.8.5 Malaysia
9.5.8.6 Thailand
9.5.8.7 Vietnam
9.5.8.8 The Philippines
9.5.8.9 Australia
9.5.8.10 New Zealand
9.5.8.11 Rest of APAC
9.6. Middle East & Africa Clinical Trial Management Software Market
9.6.1 Key Market Trends, Growth Factors and Opportunities
9.6.2 Top Key Companies
9.6.3 Historic and Forecasted Market Size by Segments
9.6.4 Historic and Forecasted Market Size by Deployment
9.6.4.1 Enterprise-wide CTMS
9.6.4.2 On-Site CTMS
9.6.5 Historic and Forecasted Market Size by Delivery
9.6.5.1 Web-based
9.6.5.2 Licensed Enterprise
9.6.5.3 Cloud-based
9.6.6 Historic and Forecasted Market Size by Component
9.6.6.1 Software
9.6.6.2 Services
9.6.7 Historic and Forecasted Market Size by End-User
9.6.7.1 Pharmaceutical and Biotechnology Firms
9.6.7.2 CROs
9.6.7.3 Medical Device Firms
9.6.8 Historic and Forecast Market Size by Country
9.6.8.1 Turkiye
9.6.8.2 Bahrain
9.6.8.3 Kuwait
9.6.8.4 Saudi Arabia
9.6.8.5 Qatar
9.6.8.6 UAE
9.6.8.7 Israel
9.6.8.8 South Africa
9.7. South America Clinical Trial Management Software Market
9.7.1 Key Market Trends, Growth Factors and Opportunities
9.7.2 Top Key Companies
9.7.3 Historic and Forecasted Market Size by Segments
9.7.4 Historic and Forecasted Market Size by Deployment
9.7.4.1 Enterprise-wide CTMS
9.7.4.2 On-Site CTMS
9.7.5 Historic and Forecasted Market Size by Delivery
9.7.5.1 Web-based
9.7.5.2 Licensed Enterprise
9.7.5.3 Cloud-based
9.7.6 Historic and Forecasted Market Size by Component
9.7.6.1 Software
9.7.6.2 Services
9.7.7 Historic and Forecasted Market Size by End-User
9.7.7.1 Pharmaceutical and Biotechnology Firms
9.7.7.2 CROs
9.7.7.3 Medical Device Firms
9.7.8 Historic and Forecast Market Size by Country
9.7.8.1 Brazil
9.7.8.2 Argentina
9.7.8.3 Rest of SA
Chapter 10 Analyst Viewpoint and Conclusion
10.1 Recommendations and Concluding Analysis
10.2 Potential Market Strategies
Chapter 11 Research Methodology
11.1 Research Process
11.2 Primary Research
11.3 Secondary Research
|
Global Clinical Trial Management Software Market |
|||
|
Base Year: |
2024 |
Forecast Period: |
2025-2032 |
|
Historical Data: |
2018 to 2023 |
Market Size in 2024: |
USD 2.09 Bn. |
|
Forecast Period 2024-32 CAGR: |
14.3% |
Market Size in 2032: |
USD 6.09 Bn. |
|
Segments Covered: |
By Deployment |
|
|
|
By Delivery |
|
||
|
By Component |
|
||
|
By End-User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||