Cancer Biomarkers Market Synopsis
Cancer Biomarkers Market Size Was Valued at USD 14.51 Billion in 2023, and is Projected to Reach USD 12.7 Billion by 2032, Growing at a CAGR of 42.56% From 2024-2032.
Cancer biomarkers are specific molecules or substances found in the body that indicate the presence of cancer or provide information about its characteristics. They can be proteins, genes, hormones, or other substances found in blood, urine, or tissue samples. Biomarkers are used for cancer detection, diagnosis, prognosis, treatment selection, and monitoring of disease progression. They help improve patient care by enabling earlier detection, personalized treatment approaches, and monitoring of treatment response.
- Biomarkers can be used for screening and early detection of cancer, allowing for timely intervention and treatment when the disease is in its early stages and more treatable. diagnosis of cancer by providing information about the presence of cancer, its location, subtype, and stage. They help distinguish between benign and malignant tumors and guide further diagnostic tests. Biomarkers provide prognostic information by predicting the likely course of the disease, including the risk of recurrence, metastasis, and overall survival.
- Biomarkers help guide treatment decisions by identifying specific molecular characteristics of the cancer that may respond to targeted therapies or personalized treatment approaches. They enable clinicians to choose the most effective treatment options while minimizing side effects. Biomarkers are used to monitor treatment response and disease progression over time. Changes in biomarker levels may indicate whether treatment is working, disease recurrence, or progression, allowing for adjustments in the treatment regimen as needed.
- Some biomarkers predict the likelihood of response to specific treatments or therapies. They help identify patients who are most likely to benefit from certain treatments and avoid unnecessary treatments in those who are unlikely to respond. Biomarkers play a crucial role in cancer research and drug development by identifying potential drug targets, evaluating treatment efficacy, and selecting patients for clinical trials. They help accelerate the development of new cancer therapies and improve patient outcomes.
- Biomarkers can aid in the early detection of cancer, allowing for timely intervention and potentially improving treatment outcomes. Biomarkers enable personalized treatment approaches by providing information about the molecular characteristics of a patient's cancer. This allows healthcare providers to tailor treatment plans to individual patients, maximizing efficacy and minimizing side effects. Biomarkers can be used to monitor the response to treatment and disease progression over time. Changes in biomarker levels can indicate whether treatment is working or if the cancer is advancing, helping healthcare providers make informed decisions about adjusting treatment regimens.
- Biomarkers provide valuable prognostic information about the likely course of the disease and the patient's outcome. This information helps healthcare providers and patients understand the severity of cancer and make decisions about treatment and care. Biomarkers play a crucial role in cancer research and drug development by identifying potential targets for new therapies and assessing the effectiveness of experimental treatments in clinical trials. They help accelerate the development of new cancer treatments and improve patient access to innovative therapies.
Cancer Biomarkers Market Trend Analysis
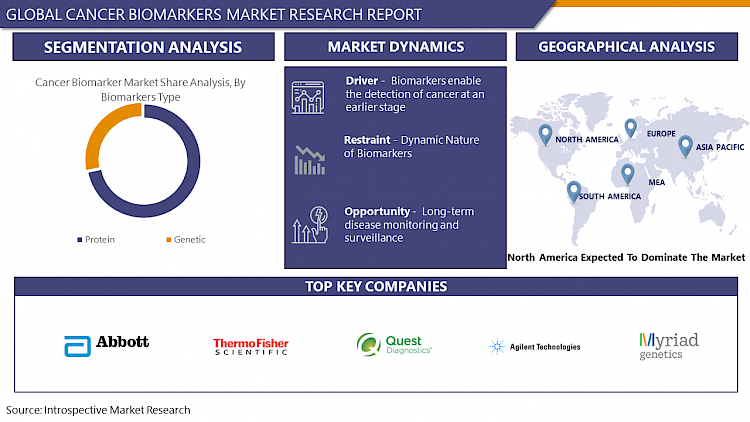
Biomarkers enable the detection of cancer at an earlier stage
- The role of biomarkers in identifying cancer in its early stages, often before symptoms manifest or conventional diagnostic methods can detect the disease. This driver is significant because early detection of cancer can lead to improved treatment outcomes and increased chances of survival for patients. Biomarkers are highly specific molecules that can be present in the body even at low concentrations, allowing for their detection in bodily fluids or tissues. By analyzing these biomarkers, healthcare providers can identify subtle changes indicative of cancer presence, often before tumors are large enough to be detected through imaging tests or physical examination.
- Biomarkers play a crucial role in cancer screening programs aimed at identifying individuals at higher risk of developing cancer. Screening programs for cancers such as breast, colorectal, prostate, and ovarian cancer often utilize biomarkers to improve early detection rates. Biomarkers are utilized in diagnostic tests to confirm or rule out the presence of cancer in patients with suspicious symptoms or abnormal screening results. These tests may involve analyzing blood, urine, or tissue samples for specific biomarkers associated with different types of cancer. By detecting cancer at an early stage, healthcare providers can initiate treatment promptly, leading to better outcomes for patients.
- Biomarkers are also used to monitor individuals at high risk of developing cancer due to factors such as family history, genetic predisposition, or exposure to carcinogens. Regular monitoring of biomarker levels allows for early detection of cancer or precancerous changes, enabling timely intervention to prevent disease progression. Advances in technology, such as molecular profiling techniques and high-throughput screening platforms, have expanded the repertoire of biomarkers available for early cancer detection. These technologies allow for the identification of novel biomarkers with high sensitivity and specificity, enhancing the accuracy of early detection methods.
Restraints
Dynamic Nature of Biomarkers
- The dynamic nature of biomarkers refers to the fact that biomarker levels or characteristics can change over time in response to various factors such as disease progression, treatment, lifestyle factors, and environmental influences. This dynamic nature presents challenges and limitations in the use of biomarkers for cancer diagnosis, prognosis, and treatment monitoring. Biomarker levels may fluctuate as cancer progresses or regresses.
- Cancer treatments such as chemotherapy, radiation therapy, or targeted therapy can impact biomarker levels. Treatment may lead to changes in biomarker expression, either directly by affecting cancer cells or indirectly by altering the tumor microenvironment or immune response. Biomarker levels can be influenced by individual patient characteristics, including age, sex, genetics, lifestyle factors, comorbidities, and medication use. These factors can contribute to inter-individual variability in biomarker expression and response to treatment, necessitating personalized approaches to biomarker analysis and interpretation.
- Tumors are often heterogeneous, consisting of diverse cell populations with distinct molecular profiles and biomarker expression patterns. This intra-tumoral heterogeneity can lead to variability in biomarker levels within the same tumor or among different metastatic sites, complicating biomarker analysis and interpretation. Advances in research and technology continually expand our understanding of cancer biology and identify novel biomarkers with diagnostic, prognostic, or predictive utility. The evolving landscape of biomarker discovery and validation necessitates ongoing evaluation and validation of biomarkers to ensure their clinical utility and relevance.
Opportunity
Long-term disease monitoring and surveillance
- Cancer biomarkers offer a promising solution for long-term disease monitoring and surveillance. These biomarkers enable healthcare providers to track disease progression, assess treatment efficacy, and detect recurrence or metastasis at early stages, leading to timely interventions and improved patient outcomes. Early detection of recurrence is possible through regular testing, allowing healthcare providers to identify signs of disease recurrence before clinical symptoms appear. Personalized treatment monitoring is also possible, as biomarkers provide real-time information on tumor response to therapy, allowing healthcare providers to adjust treatment regimens accordingly.
- Long-term monitoring of biomarkers also provides valuable prognostic information, helping to identify patients at higher risk of recurrence or metastasis and tailor surveillance strategies. Regular monitoring of high-risk populations can aid in early detection and surveillance of potential cancer development. Advances in technology and remote monitoring platforms enable the integration of biomarker testing into telemedicine and remote healthcare services. Long-term monitoring of cancer biomarkers contributes to the development of novel biomarkers, diagnostic technologies, and treatment strategies.
Challenges
Ensuring the stability and integrity of biomarkers
- The stability and integrity of cancer biomarkers are crucial in biomarker research and clinical practice. Factors such as sample collection and processing, storage conditions, freeze-thaw cycles, pre-analytical variables, biomarker assay techniques, matrix effects, and quality control measures contribute to the complexity of maintaining biomarker stability. Proper collection and processing of biological samples, such as blood, tissue, urine, or saliva, are essential for preserving biomarker integrity. Improper storage conditions can lead to degradation, loss of activity, or alteration of molecular structure, affecting the accuracy and reliability of biomarker measurements.
- Repeated freeze-thaw cycles can compromise biomarker stability, leading to denaturation, aggregation, or degradation. Standardizing pre-analytical protocols and minimizing processing delays are essential for ensuring consistent and reliable biomarker results. Matrix effects, which involve complex mixtures of biomolecules, metabolites, and contaminants, can also affect biomarker measurements. Implementing robust quality control measures is essential for maintaining biomarker assay integrity.
Cancer Biomarkers Market Segment Analysis:
Cancer Biomarkers Market Segmented based on biomarker type, profiling technology, cancer type, and application.
By biomarker type, Protein segment is expected to dominate the market during the forecast period
- Protein biomarkers are extensively studied and validated for their clinical relevance in cancer detection, diagnosis, prognosis, and treatment monitoring. Many protein biomarkers, such as PSA for prostate cancer and CA-125 for ovarian cancer, are widely used in clinical practice and guidelines. Protein biomarkers are abundant in biological fluids such as blood, serum, plasma, urine, and tissue samples, making them readily accessible for detection and measurement using various analytical techniques. This wide availability facilitates biomarker testing in routine clinical practice and research settings.
- Several well-established assay technologies, including immunoassays, enzyme-linked immunosorbent assays (ELISA), and mass spectrometry, are available for the detection and quantification of protein biomarkers. These assay technologies offer high sensitivity, specificity, and reproducibility, enhancing the reliability of protein biomarker measurements. Protein biomarkers can often be detected in minimally invasive or non-invasive samples, such as blood or urine, avoiding the need for invasive procedures such as tissue biopsies. Non-invasive sampling methods are preferred by patients and healthcare providers and enable serial monitoring of biomarker levels over time.
- Multiplex assay platforms allow simultaneous detection and quantification of multiple protein biomarkers in a single sample, providing comprehensive information about disease status and molecular profiles. Multiplexing capabilities enhance the efficiency and cost-effectiveness of biomarker testing, particularly in high-throughput screening applications. Protein biomarkers play a crucial role in translational research, facilitating the discovery and development of novel cancer diagnostics, prognostics, and therapeutics. Biomarker research drives innovation in precision medicine and personalized cancer care by identifying molecular signatures associated with specific cancer subtypes or treatment responses.
By Profiling Technology, Omics segment held the largest share of 39.3% in 2023
- Omics technologies, including genomics, proteomics, metabolomics, and transcriptomics, provide a comprehensive analysis of molecular components in cancer cells and tissues. These technologies offer high-throughput capabilities, allowing researchers and clinicians to identify novel biomarkers, characterize disease subtypes, and conduct large-scale biomarker discovery and validation studies. Molecular profiling of cancer cells, tissues, and bodily fluids provides insights into the underlying molecular mechanisms driving cancer development, progression, and treatment response.
- The information can guide personalized cancer treatment strategies, guiding the selection of targeted therapies, immunotherapies, or combination regimens. Omics technologies also play a critical role in biomarker discovery, identifying novel biomarker candidates associated with cancer diagnosis, prognosis, and treatment response. Translational research enables the translation of scientific discoveries into clinically relevant biomarker assays and diagnostic tests. Collaborative initiatives, such as research consortia, public-private partnerships, and academic-industry collaborations, drive innovation and advancement in omics technologies for cancer biomarker research.
Cancer Biomarkers Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast Period
- North America, is a global leader in cancer research and innovation, with advanced healthcare infrastructure, strong R&D capabilities, and a high prevalence of cancer. This has led to increased demand for improved cancer diagnostics, prognostics, and personalized treatment approaches. The regulatory environment in North America, particularly the U.S., is favorable for biomarker development and commercialization, with agencies like the FDA providing clear pathways for validation and approval.
- The region is home to leading companies in the field of cancer biomarkers, including diagnostic companies, biotechnology firms, and pharmaceutical companies, which leverage their expertise to develop and commercialize novel biomarker-based products for cancer detection, diagnosis, and treatment. The reimbursement landscape in North America supports the adoption of biomarker-based diagnostic tests and companion diagnostics, as payers recognize the value of biomarker testing in guiding treatment decisions and improving patient outcomes.
Cancer Biomarkers Market Top Key Players:
- Abbott Laboratories (US)
- Thermo Fisher Scientific Inc. (US)
- Quest Diagnostics (US)
- Guardant Health (US)
- Bio-Rad Laboratories, Inc. (US)
- Agilent Technologies, Inc. (US)
- Myriad Genetics, Inc. (US)
- Hologic, Inc. (US)
- PerkinElmer, Inc. (US)
- Genomic Health, Inc. (US)
- Guardant Health, Inc. (US)
- Exact Sciences Corporation (US)
- Foundation Medicine, Inc. (US)
- NanoString Technologies, Inc. (US)
- Cepheid (US)
- Siemens Healthineers AG (Germany)
- Merck KGaA (Germany)
- BioMérieux SA (France)
- Genomic Vision (France)
- DiaSorin S.p.A. (Italy)
- Biocartis NV (Belgium)
- Ibex Medical Analytics (Israel)
- Roche Diagnostics (Switzerland)
- Sysmex Corporation (Japan), and other major players
Key Industry Developments in the Cancer Biomarkers Market:
- In September 2023, Ibex launched Galen Breast HER2, to support improved biomarker scoring in breast cancer patients.
- In July 2023, Quest Diagnostics in collaboration with Envision introduced a new prostate cancer biomarker test to meet the critical clinical requirement for identifying and distinguishing potentially aggressive prostate cancer cases in men.
- In February 2023, Guardant Health entered into a partnership with Lunit to enhance cancer biomarker detection.
|
Global Cancer Biomarkers Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
14.51 Bn |
|
Forecast Period 2024-32 CAGR: |
12.7 % |
Market Size in 2032: |
42.56 Bn |
|
Segments Covered: |
By Biomarkers Type |
|
|
|
By Profiling Technology |
|
||
|
By Cancer Type |
|
||
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
- INTRODUCTION
- RESEARCH OBJECTIVES
- RESEARCH METHODOLOGY
- RESEARCH PROCESS
- SCOPE AND COVERAGE
- Market Definition
- Key Questions Answered
- MARKET SEGMENTATION
- EXECUTIVE SUMMARY
- MARKET OVERVIEW
- GROWTH OPPORTUNITIES BY SEGMENT
- MARKET LANDSCAPE
- PORTER’S FIVE FORCES ANALYSIS
- Bargaining Power Of Supplier
- Threat Of New Entrants
- Threat Of Substitutes
- Competitive Rivalry
- Bargaining Power Among Buyers
- INDUSTRY VALUE CHAIN ANALYSIS
- MARKET DYNAMICS
- Drivers
- Restraints
- Opportunities
- Challenges
- MARKET TREND ANALYSIS
- REGULATORY LANDSCAPE
- PESTLE ANALYSIS
- PRICE TREND ANALYSIS
- PATENT ANALYSIS
- TECHNOLOGY EVALUATION
- MARKET IMPACT OF THE RUSSIA-UKRAINE WAR
- Geopolitical Market Disruptions
- Supply Chain Disruptions
- Instability in Emerging Markets
- ECOSYSTEM
- PORTER’S FIVE FORCES ANALYSIS
- CANCER BIOMARKERS MARKET BY BIOMARKERS TYPE (2017-2032)
- CANCER BIOMARKERS MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- PROTEIN
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- GENETIC
- CANCER BIOMARKERS MARKET BY PROFILING TECHNOLOGY (2017-2032)
- CANCER BIOMARKERS MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- OMICS
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- IMAGING
- IMMUNOASSAYS
- CYTOGENETICS
- BIOINFORMATICS
- CANCER BIOMARKERS MARKET BY CANCER TYPE (2017-2032)
- CANCER BIOMARKERS MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- BREAST
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- ORAL
- LUNG
- PROSTATE
- STOMACH
- SKIN
- LIVER
- OTHERS
- CANCER BIOMARKERS MARKET BY APPLICATION (2017-2032)
- CANCER BIOMARKERS MARKET SNAPSHOT AND GROWTH ENGINE
- MARKET OVERVIEW
- PROGNOSTICS
- Introduction And Market Overview
- Historic And Forecasted Market Size in Value (2017 – 2032F)
- Historic And Forecasted Market Size in Volume (2017 – 2032F)
- Key Market Trends, Growth Factors And Opportunities
- Geographic Segmentation Analysis
- DIAGNOSTICS
- RESEARCH AND DEVELOPMENT
- COMPANY PROFILES AND COMPETITIVE ANALYSIS
- COMPETITIVE LANDSCAPE
- Competitive Positioning
- Cancer Biomarkers Market Share By Manufacturer (2023)
- Industry BCG Matrix
- Heat Map Analysis
- Mergers & Acquisitions
- ABBOTT LABORATORIES (US)
- Company Overview
- Key Executives
- Company Snapshot
- Role of the Company in the Market
- Sustainability and Social Responsibility
- Operating Business Segments
- Product Portfolio
- Business Performance (Production Volume, Sales Volume, Sales Margin, Production Capacity, Capacity Utilization Rate)
- Key Strategic Moves And Recent Developments
- SWOT Analysis
- THERMO FISHER SCIENTIFIC INC. (US)
- QUEST DIAGNOSTICS (US)
- GUARDANT HEALTH (US)
- BIO-RAD LABORATORIES, INC. (US)
- AGILENT TECHNOLOGIES, INC. (US)
- MYRIAD GENETICS, INC. (US)
- HOLOGIC, INC. (US)
- PERKINELMER, INC. (US)
- GENOMIC HEALTH, INC. (US)
- GUARDANT HEALTH, INC. (US)
- EXACT SCIENCES CORPORATION (US)
- FOUNDATION MEDICINE, INC. (US)
- NANOSTRING TECHNOLOGIES, INC. (US)
- CEPHEID (US)
- SIEMENS HEALTHINEERS AG (GERMANY)
- MERCK KGAA (GERMANY)
- BIOMÉRIEUX SA (FRANCE)
- GENOMIC VISION (FRANCE)
- DIASORIN S.P.A. (ITALY)
- BIOCARTIS NV (BELGIUM)
- IBEX MEDICAL ANALYTICS (ISRAEL)
- ROCHE DIAGNOSTICS (SWITZERLAND)
- SYSMEX CORPORATION (JAPAN)
- COMPETITIVE LANDSCAPE
- GLOBAL CANCER BIOMARKERS MARKET BY REGION
- OVERVIEW
- NORTH AMERICA
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Biomarkers Type
- Historic And Forecasted Market Size By Profiling Technology
- Historic And Forecasted Market Size By Cancer Type
- Historic And Forecasted Market Size By Application
- Historic And Forecasted Market Size By Country
- USA
- Canada
- Mexico
- EASTERN EUROPE
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Russia
- Bulgaria
- The Czech Republic
- Hungary
- Poland
- Romania
- Rest Of Eastern Europe
- WESTERN EUROPE
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Germany
- United Kingdom
- France
- The Netherlands
- Italy
- Spain
- Rest Of Western Europe
- ASIA PACIFIC
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Vietnam
- The Philippines
- Australia
- New-Zealand
- Rest Of APAC
- MIDDLE EAST & AFRICA
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Turkey
- Bahrain
- Kuwait
- Saudi Arabia
- Qatar
- UAE
- Israel
- South Africa
- SOUTH AMERICA
- Key Market Trends, Growth Factors And Opportunities
- Key Manufacturers
- Historic And Forecasted Market Size By Segments
- Historic And Forecasted Market Size By Country
- Brazil
- Argentina
- Rest of South America
- INVESTMENT ANALYSIS
- ANALYST VIEWPOINT AND CONCLUSION
- Recommendations and Concluding Analysis
- Potential Market Strategies
|
Global Cancer Biomarkers Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
14.51 Bn |
|
Forecast Period 2024-32 CAGR: |
12.7 % |
Market Size in 2032: |
42.56 Bn |
|
Segments Covered: |
By Biomarkers Type |
|
|
|
By Profiling Technology |
|
||
|
By Cancer Type |
|
||
|
By Application |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
LIST OF TABLES
TABLE 001. EXECUTIVE SUMMARY
TABLE 002. CANCER BIOMARKERS MARKET BARGAINING POWER OF SUPPLIERS
TABLE 003. CANCER BIOMARKERS MARKET BARGAINING POWER OF CUSTOMERS
TABLE 004. CANCER BIOMARKERS MARKET COMPETITIVE RIVALRY
TABLE 005. CANCER BIOMARKERS MARKET THREAT OF NEW ENTRANTS
TABLE 006. CANCER BIOMARKERS MARKET THREAT OF SUBSTITUTES
TABLE 007. CANCER BIOMARKERS MARKET BY TYPE
TABLE 008. NEUROSTIMULATION MARKET OVERVIEW (2016-2028)
TABLE 009. IMAGING MODALITIES MARKET OVERVIEW (2016-2028)
TABLE 010. NEUROLOGICAL IMPLANTS MARKET OVERVIEW (2016-2028)
TABLE 011. CRANIAL SURFACE MEASUREMENT MARKET OVERVIEW (2016-2028)
TABLE 012. OTHERS MARKET OVERVIEW (2016-2028)
TABLE 013. CANCER BIOMARKERS MARKET BY APPLICATION
TABLE 014. DIAGNOSTIC CENTERS MARKET OVERVIEW (2016-2028)
TABLE 015. AMBULATORY SURGICAL CENTERS MARKET OVERVIEW (2016-2028)
TABLE 016. HOSPITALS MARKET OVERVIEW (2016-2028)
TABLE 017. CLINICS MARKET OVERVIEW (2016-2028)
TABLE 018. OTHERS MARKET OVERVIEW (2016-2028)
TABLE 019. NORTH AMERICA CANCER BIOMARKERS MARKET, BY TYPE (2016-2028)
TABLE 020. NORTH AMERICA CANCER BIOMARKERS MARKET, BY APPLICATION (2016-2028)
TABLE 021. N CANCER BIOMARKERS MARKET, BY COUNTRY (2016-2028)
TABLE 022. EUROPE CANCER BIOMARKERS MARKET, BY TYPE (2016-2028)
TABLE 023. EUROPE CANCER BIOMARKERS MARKET, BY APPLICATION (2016-2028)
TABLE 024. CANCER BIOMARKERS MARKET, BY COUNTRY (2016-2028)
TABLE 025. ASIA PACIFIC CANCER BIOMARKERS MARKET, BY TYPE (2016-2028)
TABLE 026. ASIA PACIFIC CANCER BIOMARKERS MARKET, BY APPLICATION (2016-2028)
TABLE 027. CANCER BIOMARKERS MARKET, BY COUNTRY (2016-2028)
TABLE 028. MIDDLE EAST & AFRICA CANCER BIOMARKERS MARKET, BY TYPE (2016-2028)
TABLE 029. MIDDLE EAST & AFRICA CANCER BIOMARKERS MARKET, BY APPLICATION (2016-2028)
TABLE 030. CANCER BIOMARKERS MARKET, BY COUNTRY (2016-2028)
TABLE 031. SOUTH AMERICA CANCER BIOMARKERS MARKET, BY TYPE (2016-2028)
TABLE 032. SOUTH AMERICA CANCER BIOMARKERS MARKET, BY APPLICATION (2016-2028)
TABLE 033. CANCER BIOMARKERS MARKET, BY COUNTRY (2016-2028)
TABLE 034. BIOMÉRIEUX INC.: SNAPSHOT
TABLE 035. BIOMÉRIEUX INC.: BUSINESS PERFORMANCE
TABLE 036. BIOMÉRIEUX INC.: PRODUCT PORTFOLIO
TABLE 037. BIOMÉRIEUX INC.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 037. INOVIQ: SNAPSHOT
TABLE 038. INOVIQ: BUSINESS PERFORMANCE
TABLE 039. INOVIQ: PRODUCT PORTFOLIO
TABLE 040. INOVIQ: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 040. BIO-RAD LABORATORIES: SNAPSHOT
TABLE 041. BIO-RAD LABORATORIES: BUSINESS PERFORMANCE
TABLE 042. BIO-RAD LABORATORIES: PRODUCT PORTFOLIO
TABLE 043. BIO-RAD LABORATORIES: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 043. INC.: SNAPSHOT
TABLE 044. INC.: BUSINESS PERFORMANCE
TABLE 045. INC.: PRODUCT PORTFOLIO
TABLE 046. INC.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 046. ABBOTT: SNAPSHOT
TABLE 047. ABBOTT: BUSINESS PERFORMANCE
TABLE 048. ABBOTT: PRODUCT PORTFOLIO
TABLE 049. ABBOTT: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 049. BECTON: SNAPSHOT
TABLE 050. BECTON: BUSINESS PERFORMANCE
TABLE 051. BECTON: PRODUCT PORTFOLIO
TABLE 052. BECTON: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 052. DICKINSON & COMPANY: SNAPSHOT
TABLE 053. DICKINSON & COMPANY: BUSINESS PERFORMANCE
TABLE 054. DICKINSON & COMPANY: PRODUCT PORTFOLIO
TABLE 055. DICKINSON & COMPANY: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 055. MERCK KGAA: SNAPSHOT
TABLE 056. MERCK KGAA: BUSINESS PERFORMANCE
TABLE 057. MERCK KGAA: PRODUCT PORTFOLIO
TABLE 058. MERCK KGAA: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 058. QIAGEN: SNAPSHOT
TABLE 059. QIAGEN: BUSINESS PERFORMANCE
TABLE 060. QIAGEN: PRODUCT PORTFOLIO
TABLE 061. QIAGEN: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 061. THERMO FISHER SCIENTIFIC INC.: SNAPSHOT
TABLE 062. THERMO FISHER SCIENTIFIC INC.: BUSINESS PERFORMANCE
TABLE 063. THERMO FISHER SCIENTIFIC INC.: PRODUCT PORTFOLIO
TABLE 064. THERMO FISHER SCIENTIFIC INC.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 064. CENTOGENE N.V.: SNAPSHOT
TABLE 065. CENTOGENE N.V.: BUSINESS PERFORMANCE
TABLE 066. CENTOGENE N.V.: PRODUCT PORTFOLIO
TABLE 067. CENTOGENE N.V.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 067. PERKINELMER INC.: SNAPSHOT
TABLE 068. PERKINELMER INC.: BUSINESS PERFORMANCE
TABLE 069. PERKINELMER INC.: PRODUCT PORTFOLIO
TABLE 070. PERKINELMER INC.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 070. SIEMENS HEALTHCARE PRIVATE LIMITED: SNAPSHOT
TABLE 071. SIEMENS HEALTHCARE PRIVATE LIMITED: BUSINESS PERFORMANCE
TABLE 072. SIEMENS HEALTHCARE PRIVATE LIMITED: PRODUCT PORTFOLIO
TABLE 073. SIEMENS HEALTHCARE PRIVATE LIMITED: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 073. OTHER MAJOR PLAYERS: SNAPSHOT
TABLE 074. OTHER MAJOR PLAYERS: BUSINESS PERFORMANCE
TABLE 075. OTHER MAJOR PLAYERS: PRODUCT PORTFOLIO
TABLE 076. OTHER MAJOR PLAYERS: KEY STRATEGIC MOVES AND DEVELOPMENTS
LIST OF FIGURES
FIGURE 001. YEARS CONSIDERED FOR ANALYSIS
FIGURE 002. SCOPE OF THE STUDY
FIGURE 003. CANCER BIOMARKERS MARKET OVERVIEW BY REGIONS
FIGURE 004. PORTER'S FIVE FORCES ANALYSIS
FIGURE 005. BARGAINING POWER OF SUPPLIERS
FIGURE 006. COMPETITIVE RIVALRYFIGURE 007. THREAT OF NEW ENTRANTS
FIGURE 008. THREAT OF SUBSTITUTES
FIGURE 009. VALUE CHAIN ANALYSIS
FIGURE 010. PESTLE ANALYSIS
FIGURE 011. CANCER BIOMARKERS MARKET OVERVIEW BY TYPE
FIGURE 012. NEUROSTIMULATION MARKET OVERVIEW (2016-2028)
FIGURE 013. IMAGING MODALITIES MARKET OVERVIEW (2016-2028)
FIGURE 014. NEUROLOGICAL IMPLANTS MARKET OVERVIEW (2016-2028)
FIGURE 015. CRANIAL SURFACE MEASUREMENT MARKET OVERVIEW (2016-2028)
FIGURE 016. OTHERS MARKET OVERVIEW (2016-2028)
FIGURE 017. CANCER BIOMARKERS MARKET OVERVIEW BY APPLICATION
FIGURE 018. DIAGNOSTIC CENTERS MARKET OVERVIEW (2016-2028)
FIGURE 019. AMBULATORY SURGICAL CENTERS MARKET OVERVIEW (2016-2028)
FIGURE 020. HOSPITALS MARKET OVERVIEW (2016-2028)
FIGURE 021. CLINICS MARKET OVERVIEW (2016-2028)
FIGURE 022. OTHERS MARKET OVERVIEW (2016-2028)
FIGURE 023. NORTH AMERICA CANCER BIOMARKERS MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 024. EUROPE CANCER BIOMARKERS MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 025. ASIA PACIFIC CANCER BIOMARKERS MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 026. MIDDLE EAST & AFRICA CANCER BIOMARKERS MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 027. SOUTH AMERICA CANCER BIOMARKERS MARKET OVERVIEW BY COUNTRY (2016-2028)
Frequently Asked Questions :
The forecast period in the Cancer Biomarkers Market research report is 2024-2032.
Abbott Laboratories (US), Thermo Fisher Scientific Inc. (US), Quest Diagnostics (US), Guardant Health (US), Bio-Rad Laboratories, Inc. (US), Agilent Technologies, Inc. (US), Myriad Genetics, Inc. (US), Hologic, Inc. (US), PerkinElmer, Inc. (US), Genomic Health, Inc. (US), Guardant Health, Inc. (US), Exact Sciences Corporation (US), Foundation Medicine, Inc. (US), NanoString Technologies, Inc. (US), Cepheid (US), Siemens Healthineers AG (Germany), Merck KGaA (Germany), BioMérieux SA (France), Genomic Vision (France),Qiagen N.V. (Netherlands), DiaSorin S.p.A. (Italy), Biocartis NV (Belgium), Ibex Medical Analytics (Israel), Roche Diagnostics (Switzerland), Sysmex Corporation (Japan), and Other Major Players.
The Cancer Biomarkers Market is segmented into Biomarkers Type, Profiling Technology, Cancer Type, and Region. By biomarker type, the market is categorized into Protein and genetic. By Profiling Technology, the market is categorized into Omics, Imaging, Immunoassays, Cytogenetics, and Bioinformatics. By Application, the market is categorized into Prognostics, Diagnostics, Research, and Development. By region, it is analyzed across North America (U.S.; Canada; Mexico), Eastern Europe (Bulgaria; The Czech Republic; Hungary; Poland; Romania; Rest of Eastern Europe), Western Europe (Germany; UK; France; Netherlands; Italy; Russia; Spain; Rest of Western Europe), Asia-Pacific (China; India; Japan; Southeast Asia, etc.), South America (Brazil; Argentina, etc.), Middle East & Africa (Saudi Arabia; South Africa, etc.).
The Cancer Biomarkers Market refers to the global industry involved in the development, production, and commercialization of biomarkers used in the detection, diagnosis, prognosis, treatment selection, and monitoring of cancer. This market encompasses a wide range of biomarkers, including proteins, genes, circulating tumor cells, circulating nucleic acids, metabolites, and imaging biomarkers, among others. Biomarkers are used in various clinical settings, including hospitals, diagnostic laboratories, research institutions, and pharmaceutical companies, to improve cancer detection and management.
Cancer Biomarkers Market Size Was Valued at USD 14.51 Billion in 2023, and is Projected to Reach USD 12.7 Billion by 2032, Growing at a CAGR of 42.56% From 2024-2032.


































