Global Human Combination Vaccines (HCV) Market Overview
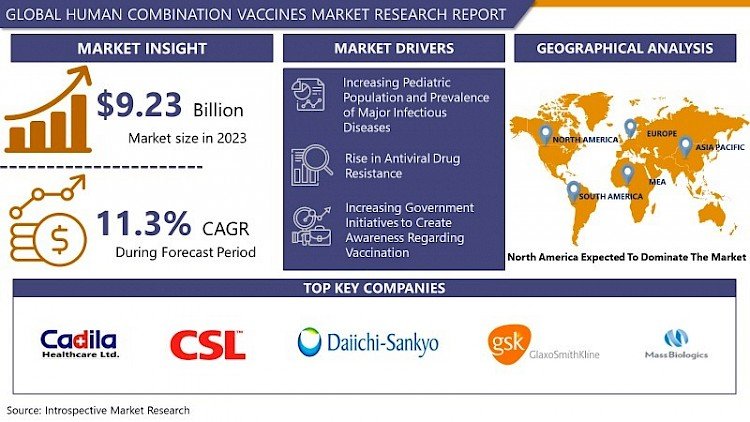
The Global Human Combination Vaccines market was valued at USD 9.23 billion in 2023 and is expected to reach USD 24.19 billion by 2032, at a CAGR of 11.3% between 2024 and 2032.
The Human Combination Vaccines Market is experiencing significant growth, driven by increasing awareness of immunization benefits, rising prevalence of infectious diseases, and the convenience of combination vaccines, which reduce the number of injections required. These vaccines protect against multiple diseases with a single shot, improving compliance rates, particularly in pediatric populations.
The market is segmented based on type, including inactivated and live attenuated vaccines. Inactivated combination vaccines dominate due to their stability and safety profile, although live attenuated vaccines offer strong immune responses with longer-lasting protection.
Geographically, North America leads the market owing to robust healthcare infrastructure, high vaccination rates, and supportive government policies. Europe follows, supported by similar factors and strong research and development (R&D) capabilities. The Asia-Pacific region is poised for substantial growth due to increasing healthcare expenditure, improving vaccine coverage, and growing awareness about immunization programs.
Key players in the market include GlaxoSmithKline, Sanofi Pasteur, Merck & Co., and Pfizer, which focus on strategic collaborations, mergers, acquisitions, and extensive R&D activities to maintain their competitive edge. Innovations in vaccine formulations and delivery methods, such as needle-free injectors and oral vaccines, are anticipated to further drive market growth.
Market Dynamics And Factors For Human Combination Vaccines Market
Drivers:
Rising Prevalence of Infectious Diseases & Increase in Novel Vaccines by Manufacturers
The rapid rise in the prevalence of infectious diseases as well as the increasing pediatric population are the major factors driving the huge growth of the human combination vaccines market. Since babies do not have a fully developed immune system during their early stages of life they are more vulnerable to many infectious diseases. For instance, the Centres for Disease Control and Prevention (CDC) reported that approximately 3.1% of the children population in the age group 5-11 years was in fair or poor health in the U.S in 2018. This thereby leads to an increase in the demand for combination vaccines thus propelling the market growth. Also, an increase in antiviral drug resistance has changed the market trend toward combination vaccines as these vaccines help reduce the problem of drug resistance.
For instance, according to a review article published in Nature, a scientific journal in 2021, appropriate utilization of vaccines reduces the spread of antimicrobial drug resistance as they are used specifically for a given infectious antigen. Moreover, the growing interest of manufacturers in developing novel vaccines to target a wide range of diseases drives the growth potential of the human combination vaccine market. For instance, in December 2017, the Government of Bangladesh collaborated with WHO (World Health Organization), Gavi the Vaccine Alliance, and UNICEF (United Nations Children’s Emergency Fund) for the need to launch a diphtheria vaccination campaign for young children in the age group 6 weeks to 6 years.
Further, The Serum Institute of India donated 300,000 pentavalent vaccine doses to the Government of Bangladesh for this vaccination campaign in 2017. Additionally, many countries have brought out immunization policies wherein they follow a fixed immunization schedule in order to immunize every newborn against any type of common infectious diseases such as diphtheria, polio, hepatitis, and tetanus which increase the demand for vaccines. For instance, in the U.S, the Advisory Committee on Immunization Practices (ACIP) recommended vaccination schedules for adults & children based on previous epidemiological studies. According to the National Conference of State Legislatures, the childhood vaccination rate for the year 2019 in the U.S. was approximately 95% and is thus expected to grow rapidly.
Restraints:
The Threat of Bioterrorism & Crucial Side Effects
The threat of bioterrorism and stringent regulatory policies for the approval of novel combination vaccines are the key factors that are hindering the growth of the human combination vaccine market. Bioterrorism refers to the use of biological agents such as fungus, bacteria, and viruses to disrupt human life. Since these biological agents are hard to predict, it is very difficult to control them. Bioterrorism can easily spread through the route of vaccines hence; regulatory authorities scrutinize the vaccine development process to avoid any mishaps. For instance, the U.S Food and Administration authority has been directed by the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 to protect the public against bioweapons associated with vaccines, diagnostic tools, and drugs in the U.S. The U.S. FDA has set out a multistep & complex process for assessing the safety, immunogenicity, as well as efficiency of the vaccine before approval of licensure for a particular vaccine, followed by post-approval surveillance. Some combinations of vaccines are associated with unfavorable side effects which hamper the human combination vaccine market growth.
For instance, in 2020, the Com-Cov study led by the University of Oxford, U.K revealed that when both the doses of AstraZeneca and Pfizer COVID-19 vaccines were mixed a higher proportion of the users experienced side effects such as fever, headache, and fatigue.
Moreover, factors such as poor healthcare infrastructure, lack of awareness, and facilities in low- and middle-income countries such as India, Vietnam, Nigeria, and Brazil hinder the market growth of human combination vaccines. For instance, according to a survey by the World Health Organization in 2018, nearly 19.4 million pediatric population in the world were without vaccination in recommended immunization schedule, and among these 60% population were recorded to be from low- and middle-income countries such as Pakistan, Indonesia, India, etc.
Opportunities:
New Approaches in Testing and Development are Estimated to Boost Protective and Durable Response
Increasing understanding of the human immune response to immunization has boosted the development of more efficacious products in the human vaccines market. Furthermore, strides made in computational and systems biology, coupled with the rapidly expanding scope of bioinformatics and machine learning in vaccine clinical trials, are enriching the landscape of the human vaccines market, thereby creating huge growth opportunities. New approaches that consist of system immunology are essential for improving the success rates and can act as a growth opportunity for the human combination vaccines market. Novel approaches help in augmenting protective and durable response, wherein the impact of psychosocial and cultural factors along with the overall biology plays a vital role. Some of the key product types propelling sales in the human vaccines market are conjugate, recombinant, inactivated, combination, and attenuated vaccines.
COVID-19 Impact Analysis On Human Combination Vaccines Market
The healthcare industry is growing at a high pace in the human combination vaccines market due to the novel coronavirus. Due to the outbreak of the virus the economy wasn’t stable in all the countries that were witnessing the impact of COVID-19 are now flourishing in the healthcare industry. The continuous research and development that is going on in hospitals and research centers are boosting the market growth worldwide. The ongoing research on the vaccine is also helping the human combination vaccines market grow in the country.
Segmentation Analysis Of Human Combination Vaccine Market
Type Insights:
The Inactivated attenuated vaccine segment dominates the growth in the human combination vaccines market. Major Vaccine manufacturers are working toward the advancement of existing vaccines as well as the development of novel vaccines which is giving rise to the growth of the human combination vaccines segment. Inactivated vaccine segment is further anticipated to grow rapidly over the projected period owing to its high adoption and acceptance as an efficient human combination vaccine. Additionally, the stability of inactivated vaccine makes it more suitable to be used for the treatment of infectious diseases as it can be stored and transported easily in lyophilized form. For instance, according to the Global Vaccine Market report by World Health Organization, in 2017 the global demand for the D&T vaccine (Diptheria and Tetanus) was approximately 945 million doses wherein DTwP-HepB-Hib vaccine accounted for 33% of the total D&T vaccine.
Regional Analysis Of Human Combination Vaccines Market
The Human Combination vaccines market is growing largely in the Asia-Pacific region. The Asia-Pacific region is accounted for the largest market share all across the globe. But in the forecast period, due to the high demand from the regions like China, Japan, Australia, and India, Asia-Pacific is expected to continue its rapid growth. The high mortality and morbidity of infectious diseases in the non-industrialized world, as well as industrialized nations, is a key driver of extensive R&D in the human vaccines market. For instance, nationwide immunization programs along with a pneumococcal conjugate vaccine (PCV7) in children have helped reduce the incidence of invasive pneumococcal disease. The fast growth is also due to the rise in the pediatric population and the increasing prevalence of infectious diseases such as tetanus, whooping cough, polio, diphtheria, chickenpox, measles, and smallpox. For instance, according to the Our World in Data report-2017, South Asia accounts for a majority of new tetanus cases. In 2019, the World Health Organisation merely reported that in South East Asia around 52% of deaths under the age of five happen during the neonatal period.
A boost in R&D activities and increasing technological advancements propel the market growth of human combination vaccines in North America. For instance, in 2019 three licensed DTaP combinations were readily available in North America for offering immunity against tetanus, diphtheria, and whooping cough (acellular pertussis) in children. These combination vaccines include Infanrix manufactured by GlaxoSmithKline, and Tripedia and Daptacel manufactured by Sanofi Pasteur. Also, booster combination vaccines Boostrix and Adacel are highly available for use in adults in the North American region which boosts the combination vaccine market growth in this region. Additionally, the U.S government has been actively involved in vaccine awareness initiatives which is another key factor that cushions the growth of human combination vaccines. Expanding understanding of infectious disease epidemiology has further helped companies develop a human vaccine that has played a crucial role in decreasing the global burden of infectious diseases.
Major Key Players Considered in the Market
- Sanofi (France)
- GlaxoSmithKline (GSK) (United Kingdom)
- Merck & Co. (United States)
- Pfizer (United States)
- AstraZeneca (United Kingdom)
- Johnson & Johnson (United States)
- Novartis (Switzerland)
- Serum Institute of India (India)
- CSL Limited (Australia)
- Bharat Biotech (India)
- Moderna (United States)
- Sinovac Biotech (China)
- Sinopharm (China)
- Inovio Pharmaceuticals (United States)
- BioNTech (Germany)
- Valneva (France)
- Medicago (Canada)
- Astellas Pharma (Japan)
- Takeda Pharmaceutical Company (Japan)
- Emergent BioSolutions (United States)
Key Industry Developments in the Human Combination Vaccines Market
- In March 2024, Moderna, Inc. announced at its fifth Vaccines Day event clinical and program updates demonstrating the advancement and acceleration of its mRNA pipeline. The updates include data readouts in the Company's respiratory and latent and other vaccine portfolios, as well as commercial, manufacturing and financial announcements for its vaccine business.
- In June 2024, Moderna, Inc. announced that its Phase 3 trial of mRNA-1083, an investigational combination vaccine against influenza and COVID-19, has met its primary endpoints, eliciting a higher immune response than the licensed comparator vaccines used in the trial.
|
Global Human Combination Vaccines Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 9.23 Bn. |
|
Forecast Period 2024-32 CAGR: |
11.3% |
Market Size in 2032: |
USD 24.19 Bn. |
|
Segments Covered: |
By Type |
|
|
|
By End-User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Research Objectives
1.2 Research Methodology
1.3 Research Process
1.4 Scope and Coverage
1.4.1 Market Definition
1.4.2 Key Questions Answered
1.5 Market Segmentation
Chapter 2:Executive Summary
Chapter 3:Growth Opportunities By Segment
3.1 By Type
3.2 By End-user
Chapter 4: Market Landscape
4.1 Porter's Five Forces Analysis
4.1.1 Bargaining Power of Supplier
4.1.2 Threat of New Entrants
4.1.3 Threat of Substitutes
4.1.4 Competitive Rivalry
4.1.5 Bargaining Power Among Buyers
4.2 Industry Value Chain Analysis
4.3 Market Dynamics
4.3.1 Drivers
4.3.2 Restraints
4.3.3 Opportunities
4.5.4 Challenges
4.4 Pestle Analysis
4.5 Technological Roadmap
4.6 Regulatory Landscape
4.7 SWOT Analysis
4.8 Price Trend Analysis
4.9 Patent Analysis
4.10 Analysis of the Impact of Covid-19
4.10.1 Impact on the Overall Market
4.10.2 Impact on the Supply Chain
4.10.3 Impact on the Key Manufacturers
4.10.4 Impact on the Pricing
Chapter 5: Human Combination Vaccines Market by Type
5.1 Human Combination Vaccines Market Overview Snapshot and Growth Engine
5.2 Human Combination Vaccines Market Overview
5.3 Inactivated Vaccines
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size (2016-2028F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Inactivated Vaccines: Grographic Segmentation
5.4 Live Attenuated Vaccines
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size (2016-2028F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Live Attenuated Vaccines: Grographic Segmentation
5.5 Other
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size (2016-2028F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 Other: Grographic Segmentation
Chapter 6: Human Combination Vaccines Market by End-user
6.1 Human Combination Vaccines Market Overview Snapshot and Growth Engine
6.2 Human Combination Vaccines Market Overview
6.3 Hospitals & Clinics
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size (2016-2028F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Hospitals & Clinics: Grographic Segmentation
6.4 Ambulatory Surgical Centers
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size (2016-2028F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 Ambulatory Surgical Centers: Grographic Segmentation
6.5 Others
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size (2016-2028F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 Others: Grographic Segmentation
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Positioning
7.1.2 Human Combination Vaccines Sales and Market Share By Players
7.1.3 Industry BCG Matrix
7.1.4 Ansoff Matrix
7.1.5 Human Combination Vaccines Industry Concentration Ratio (CR5 and HHI)
7.1.6 Top 5 Human Combination Vaccines Players Market Share
7.1.7 Mergers and Acquisitions
7.1.8 Business Strategies By Top Players
7.2 CADILA HEALTHCARE LTD.
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Operating Business Segments
7.2.5 Product Portfolio
7.2.6 Business Performance
7.2.7 Key Strategic Moves and Recent Developments
7.2.8 SWOT Analysis
7.3 CSL LTD.
7.4 DAIICHI SANKYO CO. LTD.
7.5 GLAXOSMITHKLINE PLC
7.6 MASS BIOLOGICS
7.7 MEIJI HOLDINGS CO. LTD.
7.8 MERCK & CO. INC.
7.9 MITSUBISHI TANABE PHARMA CORP.
7.10 SANOFI
7.11 TAKEDA PHARMACEUTICAL CO. LTD
7.12 OTHER MAJOR PLAYERS
Chapter 8: Global Human Combination Vaccines Market Analysis, Insights and Forecast, 2016-2028
8.1 Market Overview
8.2 Historic and Forecasted Market Size By Type
8.2.1 Inactivated Vaccines
8.2.2 Live Attenuated Vaccines
8.2.3 Other
8.3 Historic and Forecasted Market Size By End-user
8.3.1 Hospitals & Clinics
8.3.2 Ambulatory Surgical Centers
8.3.3 Others
Chapter 9: North America Human Combination Vaccines Market Analysis, Insights and Forecast, 2016-2028
9.1 Key Market Trends, Growth Factors and Opportunities
9.2 Impact of Covid-19
9.3 Key Players
9.4 Key Market Trends, Growth Factors and Opportunities
9.4 Historic and Forecasted Market Size By Type
9.4.1 Inactivated Vaccines
9.4.2 Live Attenuated Vaccines
9.4.3 Other
9.5 Historic and Forecasted Market Size By End-user
9.5.1 Hospitals & Clinics
9.5.2 Ambulatory Surgical Centers
9.5.3 Others
9.6 Historic and Forecast Market Size by Country
9.6.1 U.S.
9.6.2 Canada
9.6.3 Mexico
Chapter 10: Europe Human Combination Vaccines Market Analysis, Insights and Forecast, 2016-2028
10.1 Key Market Trends, Growth Factors and Opportunities
10.2 Impact of Covid-19
10.3 Key Players
10.4 Key Market Trends, Growth Factors and Opportunities
10.4 Historic and Forecasted Market Size By Type
10.4.1 Inactivated Vaccines
10.4.2 Live Attenuated Vaccines
10.4.3 Other
10.5 Historic and Forecasted Market Size By End-user
10.5.1 Hospitals & Clinics
10.5.2 Ambulatory Surgical Centers
10.5.3 Others
10.6 Historic and Forecast Market Size by Country
10.6.1 Germany
10.6.2 U.K.
10.6.3 France
10.6.4 Italy
10.6.5 Russia
10.6.6 Spain
10.6.7 Rest of Europe
Chapter 11: Asia-Pacific Human Combination Vaccines Market Analysis, Insights and Forecast, 2016-2028
11.1 Key Market Trends, Growth Factors and Opportunities
11.2 Impact of Covid-19
11.3 Key Players
11.4 Key Market Trends, Growth Factors and Opportunities
11.4 Historic and Forecasted Market Size By Type
11.4.1 Inactivated Vaccines
11.4.2 Live Attenuated Vaccines
11.4.3 Other
11.5 Historic and Forecasted Market Size By End-user
11.5.1 Hospitals & Clinics
11.5.2 Ambulatory Surgical Centers
11.5.3 Others
11.6 Historic and Forecast Market Size by Country
11.6.1 China
11.6.2 India
11.6.3 Japan
11.6.4 Singapore
11.6.5 Australia
11.6.6 New Zealand
11.6.7 Rest of APAC
Chapter 12: Middle East & Africa Human Combination Vaccines Market Analysis, Insights and Forecast, 2016-2028
12.1 Key Market Trends, Growth Factors and Opportunities
12.2 Impact of Covid-19
12.3 Key Players
12.4 Key Market Trends, Growth Factors and Opportunities
12.4 Historic and Forecasted Market Size By Type
12.4.1 Inactivated Vaccines
12.4.2 Live Attenuated Vaccines
12.4.3 Other
12.5 Historic and Forecasted Market Size By End-user
12.5.1 Hospitals & Clinics
12.5.2 Ambulatory Surgical Centers
12.5.3 Others
12.6 Historic and Forecast Market Size by Country
12.6.1 Turkey
12.6.2 Saudi Arabia
12.6.3 Iran
12.6.4 UAE
12.6.5 Africa
12.6.6 Rest of MEA
Chapter 13: South America Human Combination Vaccines Market Analysis, Insights and Forecast, 2016-2028
13.1 Key Market Trends, Growth Factors and Opportunities
13.2 Impact of Covid-19
13.3 Key Players
13.4 Key Market Trends, Growth Factors and Opportunities
13.4 Historic and Forecasted Market Size By Type
13.4.1 Inactivated Vaccines
13.4.2 Live Attenuated Vaccines
13.4.3 Other
13.5 Historic and Forecasted Market Size By End-user
13.5.1 Hospitals & Clinics
13.5.2 Ambulatory Surgical Centers
13.5.3 Others
13.6 Historic and Forecast Market Size by Country
13.6.1 Brazil
13.6.2 Argentina
13.6.3 Rest of SA
Chapter 14 Investment Analysis
Chapter 15 Analyst Viewpoint and Conclusion
|
Global Human Combination Vaccines Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 9.23 Bn. |
|
Forecast Period 2024-32 CAGR: |
11.3% |
Market Size in 2032: |
USD 24.19 Bn. |
|
Segments Covered: |
By Type |
|
|
|
By End-User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
LIST OF TABLES
TABLE 001. EXECUTIVE SUMMARY
TABLE 002. HUMAN COMBINATION VACCINES MARKET BARGAINING POWER OF SUPPLIERS
TABLE 003. HUMAN COMBINATION VACCINES MARKET BARGAINING POWER OF CUSTOMERS
TABLE 004. HUMAN COMBINATION VACCINES MARKET COMPETITIVE RIVALRY
TABLE 005. HUMAN COMBINATION VACCINES MARKET THREAT OF NEW ENTRANTS
TABLE 006. HUMAN COMBINATION VACCINES MARKET THREAT OF SUBSTITUTES
TABLE 007. HUMAN COMBINATION VACCINES MARKET BY TYPE
TABLE 008. INACTIVATED VACCINES MARKET OVERVIEW (2016-2028)
TABLE 009. LIVE ATTENUATED VACCINES MARKET OVERVIEW (2016-2028)
TABLE 010. OTHER MARKET OVERVIEW (2016-2028)
TABLE 011. HUMAN COMBINATION VACCINES MARKET BY END-USER
TABLE 012. HOSPITALS & CLINICS MARKET OVERVIEW (2016-2028)
TABLE 013. AMBULATORY SURGICAL CENTERS MARKET OVERVIEW (2016-2028)
TABLE 014. OTHERS MARKET OVERVIEW (2016-2028)
TABLE 015. NORTH AMERICA HUMAN COMBINATION VACCINES MARKET, BY TYPE (2016-2028)
TABLE 016. NORTH AMERICA HUMAN COMBINATION VACCINES MARKET, BY END-USER (2016-2028)
TABLE 017. N HUMAN COMBINATION VACCINES MARKET, BY COUNTRY (2016-2028)
TABLE 018. EUROPE HUMAN COMBINATION VACCINES MARKET, BY TYPE (2016-2028)
TABLE 019. EUROPE HUMAN COMBINATION VACCINES MARKET, BY END-USER (2016-2028)
TABLE 020. HUMAN COMBINATION VACCINES MARKET, BY COUNTRY (2016-2028)
TABLE 021. ASIA PACIFIC HUMAN COMBINATION VACCINES MARKET, BY TYPE (2016-2028)
TABLE 022. ASIA PACIFIC HUMAN COMBINATION VACCINES MARKET, BY END-USER (2016-2028)
TABLE 023. HUMAN COMBINATION VACCINES MARKET, BY COUNTRY (2016-2028)
TABLE 024. MIDDLE EAST & AFRICA HUMAN COMBINATION VACCINES MARKET, BY TYPE (2016-2028)
TABLE 025. MIDDLE EAST & AFRICA HUMAN COMBINATION VACCINES MARKET, BY END-USER (2016-2028)
TABLE 026. HUMAN COMBINATION VACCINES MARKET, BY COUNTRY (2016-2028)
TABLE 027. SOUTH AMERICA HUMAN COMBINATION VACCINES MARKET, BY TYPE (2016-2028)
TABLE 028. SOUTH AMERICA HUMAN COMBINATION VACCINES MARKET, BY END-USER (2016-2028)
TABLE 029. HUMAN COMBINATION VACCINES MARKET, BY COUNTRY (2016-2028)
TABLE 030. CADILA HEALTHCARE LTD.: SNAPSHOT
TABLE 031. CADILA HEALTHCARE LTD.: BUSINESS PERFORMANCE
TABLE 032. CADILA HEALTHCARE LTD.: PRODUCT PORTFOLIO
TABLE 033. CADILA HEALTHCARE LTD.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 033. CSL LTD.: SNAPSHOT
TABLE 034. CSL LTD.: BUSINESS PERFORMANCE
TABLE 035. CSL LTD.: PRODUCT PORTFOLIO
TABLE 036. CSL LTD.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 036. DAIICHI SANKYO CO. LTD.: SNAPSHOT
TABLE 037. DAIICHI SANKYO CO. LTD.: BUSINESS PERFORMANCE
TABLE 038. DAIICHI SANKYO CO. LTD.: PRODUCT PORTFOLIO
TABLE 039. DAIICHI SANKYO CO. LTD.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 039. GLAXOSMITHKLINE PLC: SNAPSHOT
TABLE 040. GLAXOSMITHKLINE PLC: BUSINESS PERFORMANCE
TABLE 041. GLAXOSMITHKLINE PLC: PRODUCT PORTFOLIO
TABLE 042. GLAXOSMITHKLINE PLC: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 042. MASS BIOLOGICS: SNAPSHOT
TABLE 043. MASS BIOLOGICS: BUSINESS PERFORMANCE
TABLE 044. MASS BIOLOGICS: PRODUCT PORTFOLIO
TABLE 045. MASS BIOLOGICS: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 045. MEIJI HOLDINGS CO. LTD.: SNAPSHOT
TABLE 046. MEIJI HOLDINGS CO. LTD.: BUSINESS PERFORMANCE
TABLE 047. MEIJI HOLDINGS CO. LTD.: PRODUCT PORTFOLIO
TABLE 048. MEIJI HOLDINGS CO. LTD.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 048. MERCK & CO. INC.: SNAPSHOT
TABLE 049. MERCK & CO. INC.: BUSINESS PERFORMANCE
TABLE 050. MERCK & CO. INC.: PRODUCT PORTFOLIO
TABLE 051. MERCK & CO. INC.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 051. MITSUBISHI TANABE PHARMA CORP.: SNAPSHOT
TABLE 052. MITSUBISHI TANABE PHARMA CORP.: BUSINESS PERFORMANCE
TABLE 053. MITSUBISHI TANABE PHARMA CORP.: PRODUCT PORTFOLIO
TABLE 054. MITSUBISHI TANABE PHARMA CORP.: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 054. SANOFI: SNAPSHOT
TABLE 055. SANOFI: BUSINESS PERFORMANCE
TABLE 056. SANOFI: PRODUCT PORTFOLIO
TABLE 057. SANOFI: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 057. TAKEDA PHARMACEUTICAL CO. LTD: SNAPSHOT
TABLE 058. TAKEDA PHARMACEUTICAL CO. LTD: BUSINESS PERFORMANCE
TABLE 059. TAKEDA PHARMACEUTICAL CO. LTD: PRODUCT PORTFOLIO
TABLE 060. TAKEDA PHARMACEUTICAL CO. LTD: KEY STRATEGIC MOVES AND DEVELOPMENTS
TABLE 060. OTHER MAJOR PLAYERS: SNAPSHOT
TABLE 061. OTHER MAJOR PLAYERS: BUSINESS PERFORMANCE
TABLE 062. OTHER MAJOR PLAYERS: PRODUCT PORTFOLIO
TABLE 063. OTHER MAJOR PLAYERS: KEY STRATEGIC MOVES AND DEVELOPMENTS
LIST OF FIGURES
FIGURE 001. YEARS CONSIDERED FOR ANALYSIS
FIGURE 002. SCOPE OF THE STUDY
FIGURE 003. HUMAN COMBINATION VACCINES MARKET OVERVIEW BY REGIONS
FIGURE 004. PORTER'S FIVE FORCES ANALYSIS
FIGURE 005. BARGAINING POWER OF SUPPLIERS
FIGURE 006. COMPETITIVE RIVALRYFIGURE 007. THREAT OF NEW ENTRANTS
FIGURE 008. THREAT OF SUBSTITUTES
FIGURE 009. VALUE CHAIN ANALYSIS
FIGURE 010. PESTLE ANALYSIS
FIGURE 011. HUMAN COMBINATION VACCINES MARKET OVERVIEW BY TYPE
FIGURE 012. INACTIVATED VACCINES MARKET OVERVIEW (2016-2028)
FIGURE 013. LIVE ATTENUATED VACCINES MARKET OVERVIEW (2016-2028)
FIGURE 014. OTHER MARKET OVERVIEW (2016-2028)
FIGURE 015. HUMAN COMBINATION VACCINES MARKET OVERVIEW BY END-USER
FIGURE 016. HOSPITALS & CLINICS MARKET OVERVIEW (2016-2028)
FIGURE 017. AMBULATORY SURGICAL CENTERS MARKET OVERVIEW (2016-2028)
FIGURE 018. OTHERS MARKET OVERVIEW (2016-2028)
FIGURE 019. NORTH AMERICA HUMAN COMBINATION VACCINES MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 020. EUROPE HUMAN COMBINATION VACCINES MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 021. ASIA PACIFIC HUMAN COMBINATION VACCINES MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 022. MIDDLE EAST & AFRICA HUMAN COMBINATION VACCINES MARKET OVERVIEW BY COUNTRY (2016-2028)
FIGURE 023. SOUTH AMERICA HUMAN COMBINATION VACCINES MARKET OVERVIEW BY COUNTRY (2016-2028)
Frequently Asked Questions :
The forecast period in the Human Combination Vaccines Market research report is 2024-2032.
Cadila Healthcare Ltd. (India), CSL Ltd (Australia), Daiichi Sankyo Co. Ltd (Japan), GlaxoSmithKline plc (UK), Mass Biologics (Boston), and other major players.
The Human Combination Vaccines Market is segmented into Type, End-user and region. By Type, the market is categorized into Inactivated Vaccines, Live Attenuated Vaccines, and Other. By End-user, the market is categorized into Hospitals & Clinics, Ambulatory Surgical Centers, and Others. By region, it is analyzed across North America (U.S.; Canada; Mexico), Europe (Germany; U.K.; France; Italy; Russia; Spain, etc.), Asia-Pacific (China; India; Japan; Southeast Asia, etc.), South America (Brazil; Argentina, etc.), Middle East & Africa (Saudi Arabia; South Africa, etc.).
A human combination vaccine is a product that protects against two or more diseases against a single one that is caused by an organism. The vaccines contain antigens that are combined, or it is mixed before administration.
The Global Human Combination Vaccines market was valued at USD 9.23 billion in 2023 and is expected to reach USD 24.19 billion by 2032, at a CAGR of 11.3% between 2024 and 2032.


































