Lentiviral Vectors Market Synopsis:
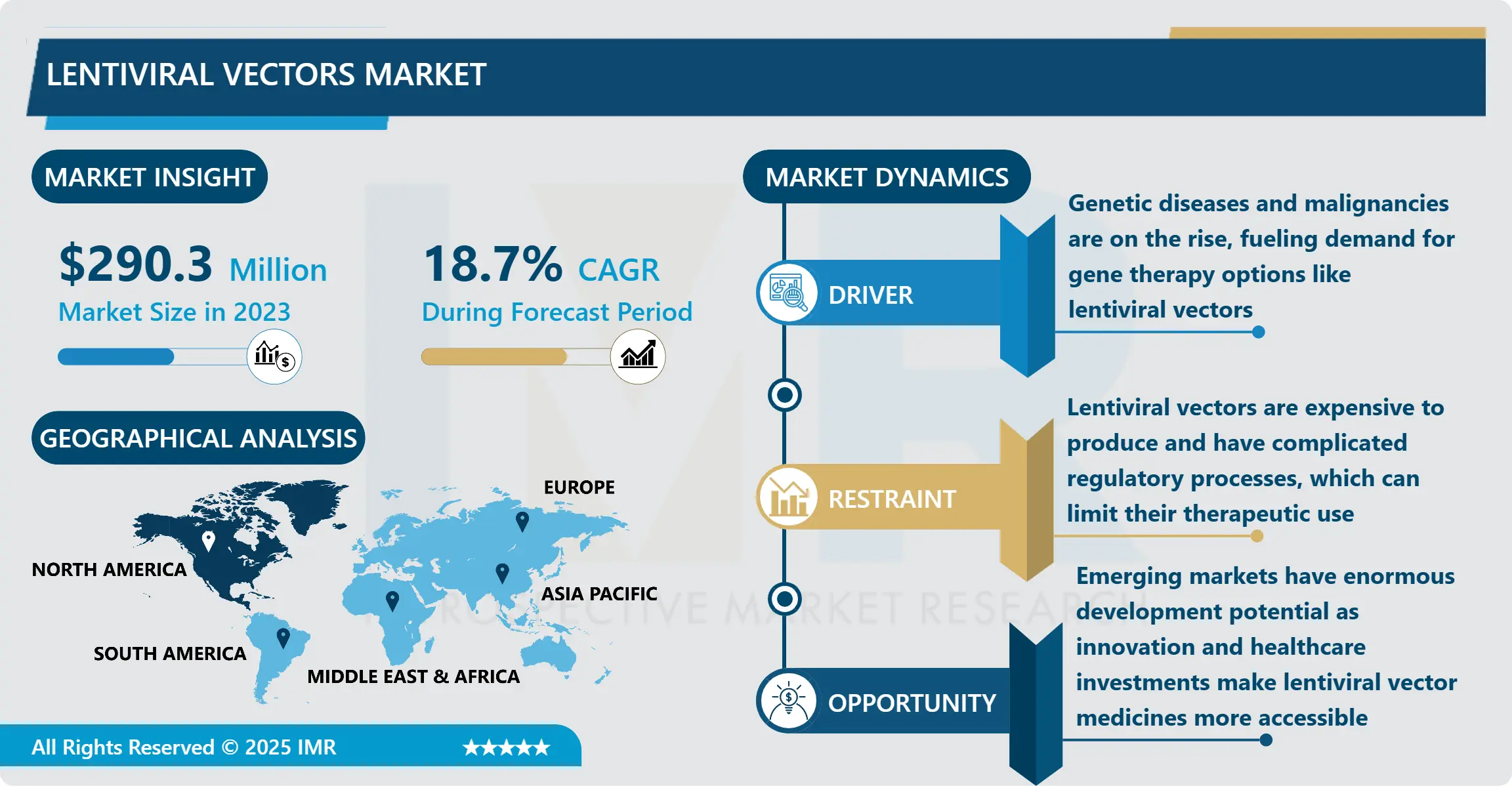
Lentiviral Vectors Market Size was valued at USD 290.3 million in 2023, and is Projected to Reach USD 1358 million by 2032, Growing at a CAGR of 18.7% From 2024-2032.
The lentiviral vectors market has seen considerable growth in recent years, driven by advancements in gene therapy and the increasing need for efficient delivery systems in genetic research and clinical applications. Lentiviral vectors, derived from the lentivirus, are particularly valuable for gene therapy due to their ability to integrate into the host genome and sustain long-term gene expression. This unique capability has led to their widespread adoption for treating genetic disorders, cancers, and other complex diseases, with the global market projected to expand at a substantial compound annual growth rate (CAGR) over the next decade.
One of the key drivers behind the lentiviral vectors market is the rise in clinical trials focused on gene and cell therapies. These vectors have demonstrated effectiveness in CAR-T cell therapies for cancer and treatments for inherited disorders such as sickle cell anemia and cystic fibrosis. With increasing investment from both private and public sectors, pharmaceutical and biotech companies are intensifying their research efforts, leading to enhanced production capacities, improved vector design, and reduced manufacturing costs. The growing pipeline of gene therapy products is expected to contribute significantly to market expansion, particularly in North America and Europe, where supportive regulatory frameworks encourage innovation.
However, challenges remain, particularly regarding the complexities of large-scale manufacturing and concerns about safety and regulatory compliance. Despite these challenges, there are growing opportunities for lentiviral vectors in emerging markets, as well as in new applications like regenerative medicine and vaccine development. Innovations in vector design to improve targeting, reduce immunogenicity, and enhance safety profiles are expected to open new avenues for growth, positioning the lentiviral vectors market as a critical component in the future of gene and cell therapy advancements.
Lentiviral Vectors Market Trend Analysis:
Increasing Adoption of Gene Therapy Applications
-
The lentiviral vectors market is witnessing a significant trend driven by the rising adoption of gene therapy for various genetic disorders and cancers. As researchers and healthcare providers increasingly focus on developing innovative therapies to treat previously incurable diseases, the demand for effective and reliable delivery systems, such as lentiviral vectors, is growing. This trend is supported by advancements in biotechnology and regulatory approvals for gene therapy products, further boosting market growth.
Advancements in Vector Engineering Technologies
-
Another notable trend in the lentiviral vectors market is the continuous advancements in vector engineering technologies. Innovations in genetic modification techniques, such as CRISPR and other gene-editing tools, are enhancing the efficacy and safety profiles of lentiviral vectors. These technological improvements are facilitating the development of more targeted and efficient therapeutic strategies, leading to an increase in research activities and clinical applications of lentiviral vectors across various fields, including oncology, neurology, and hematology.
Lentiviral Vectors Market Segment Analysis:
Lentiviral Vectors Market is Segmented on the basis of Component, Type, Generation, Workflow, Delivery Method, Disease Indication, Application, End User, and Region.
By Component, Lentiviral promoter segment is expected to dominate the market during the forecast period
-
The lentiviral vectors market can be segmented by component into lentiviral promoters, lentiviral fusion tags, lentivirus packaging systems, and other related elements. Lentiviral promoters are crucial for driving the expression of therapeutic genes, playing a significant role in the effectiveness of gene delivery. Lentiviral fusion tags facilitate the identification and purification of proteins expressed from lentiviral vectors, thereby enhancing research and therapeutic outcomes. Lentivirus packaging systems are essential for producing high-titer lentiviral vectors, ensuring efficient gene transfer and expression in target cells. The other components category includes various auxiliary materials and reagents necessary for the development and application of lentiviral vectors. This segmentation underscores the diverse applications and innovations within the market, reflecting the growing demand for tailored solutions in gene therapy and molecular research.
By Application, Gene Therapy segment expected to held the largest share
-
The lentiviral vectors market is significantly impacted by its applications in gene therapy and vaccinology, where these vectors serve as essential tools for delivering genetic material into cells. In gene therapy, lentiviral vectors are utilized to introduce therapeutic genes that can correct genetic disorders or combat diseases such as cancer, thereby offering promising treatment options. Their ability to integrate into the host genome allows for stable and long-term expression of the desired gene, enhancing treatment efficacy. Additionally, in vaccinology, lentiviral vectors are being explored as innovative vaccine delivery systems, especially for emerging infectious diseases and cancer immunotherapies. By facilitating the expression of specific antigens, these vectors can stimulate robust immune responses, making them a valuable asset in the development of next-generation vaccines. As a result, the growing interest in gene therapies and vaccine innovations continues to drive the demand for lentiviral vectors, fostering advancements in research and clinical applications.
Lentiviral Vectors Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
-
North America is expected to dominate the lentiviral vectors market over the forecast period due to a combination of robust research and development activities, a well-established biotechnology sector, and significant investments in gene therapy innovations. The region benefits from leading academic institutions and research organizations that are at the forefront of gene therapy research, driving demand for advanced delivery systems like lentiviral vectors. Additionally, supportive regulatory frameworks and funding initiatives from government bodies and private entities facilitate clinical trials and the commercialization of lentiviral-based therapies. The presence of key market players in North America further strengthens the region's position, as they focus on developing cutting-edge technologies and expanding their product offerings to meet the growing needs of the healthcare industry.
Active Key Players in the Lentiviral Vectors Market:
-
Cobra Biologics Limited (U.K.)
- Sirion-Biotech GmbH (Germany)
- Merck KGaA (Germany)
- FinVector Oy (Finland)
- Oxford Biomedica (U.K.)
- OriGene Technologies, Inc. (U.S.)
- Sino Biological Inc. (China)
- Cell Biolabs, Inc. (U.S.)
- Batavia Biosciences B.V. (Netherlands)
- Lonza (Switzerland)
- GENEMEDI (China)
- Takara Bio Inc. (Japan)
- Thermo Fisher Scientific Inc. (U.S.)
- Waisman Biomanufacturing (U.S.)
- Cytiva (U.S.)
- Other Active Players
|
Lentiviral Vectors Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 290.3 Million |
|
Forecast Period 2024-32 CAGR: |
18.7% |
Market Size in 2032: |
USD 1358 Million |
|
Segments Covered: |
By Component |
|
|
|
By Type |
|
||
|
By Generation |
|
||
|
By Workflow |
|
||
|
By Delivery Method |
|
||
|
By Disease Indication |
|
||
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter's Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Lentiviral Vectors Market by Component
4.1 Lentiviral Vectors Market Snapshot and Growth Engine
4.2 Lentiviral Vectors Market Overview
4.3 Lentiviral promoter
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 Lentiviral promoter: Geographic Segmentation Analysis
4.4 Lentiviral fusion tags
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Lentiviral fusion tags: Geographic Segmentation Analysis
4.5 Lentivirus packaging systems and Other
4.5.1 Introduction and Market Overview
4.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.5.3 Key Market Trends, Growth Factors and Opportunities
4.5.4 Lentivirus packaging systems and Other: Geographic Segmentation Analysis
Chapter 5: Lentiviral Vectors Market by Type
5.1 Lentiviral Vectors Market Snapshot and Growth Engine
5.2 Lentiviral Vectors Market Overview
5.3 Product and Services
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Product and Services: Geographic Segmentation Analysis
Chapter 6: Lentiviral Vectors Market by Generation
6.1 Lentiviral Vectors Market Snapshot and Growth Engine
6.2 Lentiviral Vectors Market Overview
6.3 4th-generation
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 4th-generation: Geographic Segmentation Analysis
6.4 3rd-generation
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 3rd-generation: Geographic Segmentation Analysis
6.5 2nd-generation and 1st-generation
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 2nd-generation and 1st-generation: Geographic Segmentation Analysis
Chapter 7: Lentiviral Vectors Market by Workflow
7.1 Lentiviral Vectors Market Snapshot and Growth Engine
7.2 Lentiviral Vectors Market Overview
7.3 Upstream Processing And Downstream Processing
7.3.1 Introduction and Market Overview
7.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
7.3.3 Key Market Trends, Growth Factors and Opportunities
7.3.4 Upstream Processing And Downstream Processing: Geographic Segmentation Analysis
Chapter 8: Lentiviral Vectors Market by Delivery Method
8.1 Lentiviral Vectors Market Snapshot and Growth Engine
8.2 Lentiviral Vectors Market Overview
8.3 In Vivo and Ex Vivo
8.3.1 Introduction and Market Overview
8.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
8.3.3 Key Market Trends, Growth Factors and Opportunities
8.3.4 In Vivo and Ex Vivo: Geographic Segmentation Analysis
Chapter 9: Lentiviral Vectors Market by Disease Indication
9.1 Lentiviral Vectors Market Snapshot and Growth Engine
9.2 Lentiviral Vectors Market Overview
9.3 Cancer
9.3.1 Introduction and Market Overview
9.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
9.3.3 Key Market Trends, Growth Factors and Opportunities
9.3.4 Cancer: Geographic Segmentation Analysis
9.4 Genetic Disorders
9.4.1 Introduction and Market Overview
9.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
9.4.3 Key Market Trends, Growth Factors and Opportunities
9.4.4 Genetic Disorders: Geographic Segmentation Analysis
9.5 Infectious Diseases
9.5.1 Introduction and Market Overview
9.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
9.5.3 Key Market Trends, Growth Factors and Opportunities
9.5.4 Infectious Diseases: Geographic Segmentation Analysis
9.6 Veterinary Disease And Other
9.6.1 Introduction and Market Overview
9.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
9.6.3 Key Market Trends, Growth Factors and Opportunities
9.6.4 Veterinary Disease And Other: Geographic Segmentation Analysis
Chapter 10: Lentiviral Vectors Market by Application
10.1 Lentiviral Vectors Market Snapshot and Growth Engine
10.2 Lentiviral Vectors Market Overview
10.3 Gene Therapy And Vaccinology
10.3.1 Introduction and Market Overview
10.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
10.3.3 Key Market Trends, Growth Factors and Opportunities
10.3.4 Gene Therapy And Vaccinology: Geographic Segmentation Analysis
Chapter 11: Lentiviral Vectors Market by End User
11.1 Lentiviral Vectors Market Snapshot and Growth Engine
11.2 Lentiviral Vectors Market Overview
11.3 Biotechnology Companies
11.3.1 Introduction and Market Overview
11.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
11.3.3 Key Market Trends, Growth Factors and Opportunities
11.3.4 Biotechnology Companies: Geographic Segmentation Analysis
11.4 Pharmaceutical Companies
11.4.1 Introduction and Market Overview
11.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
11.4.3 Key Market Trends, Growth Factors and Opportunities
11.4.4 Pharmaceutical Companies: Geographic Segmentation Analysis
11.5 Contract Research Organizations
11.5.1 Introduction and Market Overview
11.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
11.5.3 Key Market Trends, Growth Factors and Opportunities
11.5.4 Contract Research Organizations: Geographic Segmentation Analysis
11.6 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
11.6.1 Introduction and Market Overview
11.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
11.6.3 Key Market Trends, Growth Factors and Opportunities
11.6.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes: Geographic Segmentation Analysis
Chapter 12: Company Profiles and Competitive Analysis
12.1 Competitive Landscape
12.1.1 Competitive Benchmarking
12.1.2 Lentiviral Vectors Market Share by Manufacturer (2023)
12.1.3 Industry BCG Matrix
12.1.4 Heat Map Analysis
12.1.5 Mergers and Acquisitions
12.2 COBRA BIOLOGICS LIMITED (U.K.)
12.2.1 Company Overview
12.2.2 Key Executives
12.2.3 Company Snapshot
12.2.4 Role of the Company in the Market
12.2.5 Sustainability and Social Responsibility
12.2.6 Operating Business Segments
12.2.7 Product Portfolio
12.2.8 Business Performance
12.2.9 Key Strategic Moves and Recent Developments
12.2.10 SWOT Analysis
12.3 SIRION-BIOTECH GMBH (GERMANY)
12.4 MERCK KGAA (GERMANY)
12.5 FINVECTOR OY (FINLAND)
12.6 OXFORD BIOMEDICA (U.K.)
12.7 ORIGENE TECHNOLOGIES INC. (U.S.)
12.8 SINO BIOLOGICAL INC. (CHINA)
12.9 CELL BIOLABS INC. (U.S.)
12.10 BATAVIA BIOSCIENCES B.V. (NETHERLANDS)
12.11 LONZA (SWITZERLAND)
12.12 GENEMEDI (CHINA)
12.13 TAKARA BIO INC. (JAPAN)
12.14 THERMO FISHER SCIENTIFIC INC. (U.S.)
12.15 WAISMAN BIOMANUFACTURING (U.S.)
12.16 CYTIVA (U.S.)
12.17 OTHER ACTIVE PLAYERS
Chapter 13: Global Lentiviral Vectors Market By Region
13.1 Overview
13.2. North America Lentiviral Vectors Market
13.2.1 Key Market Trends, Growth Factors and Opportunities
13.2.2 Top Key Companies
13.2.3 Historic and Forecasted Market Size by Segments
13.2.4 Historic and Forecasted Market Size By Component
13.2.4.1 Lentiviral promoter
13.2.4.2 Lentiviral fusion tags
13.2.4.3 Lentivirus packaging systems and Other
13.2.5 Historic and Forecasted Market Size By Type
13.2.5.1 Product and Services
13.2.6 Historic and Forecasted Market Size By Generation
13.2.6.1 4th-generation
13.2.6.2 3rd-generation
13.2.6.3 2nd-generation and 1st-generation
13.2.7 Historic and Forecasted Market Size By Workflow
13.2.7.1 Upstream Processing And Downstream Processing
13.2.8 Historic and Forecasted Market Size By Delivery Method
13.2.8.1 In Vivo and Ex Vivo
13.2.9 Historic and Forecasted Market Size By Disease Indication
13.2.9.1 Cancer
13.2.9.2 Genetic Disorders
13.2.9.3 Infectious Diseases
13.2.9.4 Veterinary Disease And Other
13.2.10 Historic and Forecasted Market Size By Application
13.2.10.1 Gene Therapy And Vaccinology
13.2.11 Historic and Forecasted Market Size By End User
13.2.11.1 Biotechnology Companies
13.2.11.2 Pharmaceutical Companies
13.2.11.3 Contract Research Organizations
13.2.11.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
13.2.12 Historic and Forecast Market Size by Country
13.2.12.1 US
13.2.12.2 Canada
13.2.12.3 Mexico
13.3. Eastern Europe Lentiviral Vectors Market
13.3.1 Key Market Trends, Growth Factors and Opportunities
13.3.2 Top Key Companies
13.3.3 Historic and Forecasted Market Size by Segments
13.3.4 Historic and Forecasted Market Size By Component
13.3.4.1 Lentiviral promoter
13.3.4.2 Lentiviral fusion tags
13.3.4.3 Lentivirus packaging systems and Other
13.3.5 Historic and Forecasted Market Size By Type
13.3.5.1 Product and Services
13.3.6 Historic and Forecasted Market Size By Generation
13.3.6.1 4th-generation
13.3.6.2 3rd-generation
13.3.6.3 2nd-generation and 1st-generation
13.3.7 Historic and Forecasted Market Size By Workflow
13.3.7.1 Upstream Processing And Downstream Processing
13.3.8 Historic and Forecasted Market Size By Delivery Method
13.3.8.1 In Vivo and Ex Vivo
13.3.9 Historic and Forecasted Market Size By Disease Indication
13.3.9.1 Cancer
13.3.9.2 Genetic Disorders
13.3.9.3 Infectious Diseases
13.3.9.4 Veterinary Disease And Other
13.3.10 Historic and Forecasted Market Size By Application
13.3.10.1 Gene Therapy And Vaccinology
13.3.11 Historic and Forecasted Market Size By End User
13.3.11.1 Biotechnology Companies
13.3.11.2 Pharmaceutical Companies
13.3.11.3 Contract Research Organizations
13.3.11.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
13.3.12 Historic and Forecast Market Size by Country
13.3.12.1 Russia
13.3.12.2 Bulgaria
13.3.12.3 The Czech Republic
13.3.12.4 Hungary
13.3.12.5 Poland
13.3.12.6 Romania
13.3.12.7 Rest of Eastern Europe
13.4. Western Europe Lentiviral Vectors Market
13.4.1 Key Market Trends, Growth Factors and Opportunities
13.4.2 Top Key Companies
13.4.3 Historic and Forecasted Market Size by Segments
13.4.4 Historic and Forecasted Market Size By Component
13.4.4.1 Lentiviral promoter
13.4.4.2 Lentiviral fusion tags
13.4.4.3 Lentivirus packaging systems and Other
13.4.5 Historic and Forecasted Market Size By Type
13.4.5.1 Product and Services
13.4.6 Historic and Forecasted Market Size By Generation
13.4.6.1 4th-generation
13.4.6.2 3rd-generation
13.4.6.3 2nd-generation and 1st-generation
13.4.7 Historic and Forecasted Market Size By Workflow
13.4.7.1 Upstream Processing And Downstream Processing
13.4.8 Historic and Forecasted Market Size By Delivery Method
13.4.8.1 In Vivo and Ex Vivo
13.4.9 Historic and Forecasted Market Size By Disease Indication
13.4.9.1 Cancer
13.4.9.2 Genetic Disorders
13.4.9.3 Infectious Diseases
13.4.9.4 Veterinary Disease And Other
13.4.10 Historic and Forecasted Market Size By Application
13.4.10.1 Gene Therapy And Vaccinology
13.4.11 Historic and Forecasted Market Size By End User
13.4.11.1 Biotechnology Companies
13.4.11.2 Pharmaceutical Companies
13.4.11.3 Contract Research Organizations
13.4.11.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
13.4.12 Historic and Forecast Market Size by Country
13.4.12.1 Germany
13.4.12.2 UK
13.4.12.3 France
13.4.12.4 The Netherlands
13.4.12.5 Italy
13.4.12.6 Spain
13.4.12.7 Rest of Western Europe
13.5. Asia Pacific Lentiviral Vectors Market
13.5.1 Key Market Trends, Growth Factors and Opportunities
13.5.2 Top Key Companies
13.5.3 Historic and Forecasted Market Size by Segments
13.5.4 Historic and Forecasted Market Size By Component
13.5.4.1 Lentiviral promoter
13.5.4.2 Lentiviral fusion tags
13.5.4.3 Lentivirus packaging systems and Other
13.5.5 Historic and Forecasted Market Size By Type
13.5.5.1 Product and Services
13.5.6 Historic and Forecasted Market Size By Generation
13.5.6.1 4th-generation
13.5.6.2 3rd-generation
13.5.6.3 2nd-generation and 1st-generation
13.5.7 Historic and Forecasted Market Size By Workflow
13.5.7.1 Upstream Processing And Downstream Processing
13.5.8 Historic and Forecasted Market Size By Delivery Method
13.5.8.1 In Vivo and Ex Vivo
13.5.9 Historic and Forecasted Market Size By Disease Indication
13.5.9.1 Cancer
13.5.9.2 Genetic Disorders
13.5.9.3 Infectious Diseases
13.5.9.4 Veterinary Disease And Other
13.5.10 Historic and Forecasted Market Size By Application
13.5.10.1 Gene Therapy And Vaccinology
13.5.11 Historic and Forecasted Market Size By End User
13.5.11.1 Biotechnology Companies
13.5.11.2 Pharmaceutical Companies
13.5.11.3 Contract Research Organizations
13.5.11.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
13.5.12 Historic and Forecast Market Size by Country
13.5.12.1 China
13.5.12.2 India
13.5.12.3 Japan
13.5.12.4 South Korea
13.5.12.5 Malaysia
13.5.12.6 Thailand
13.5.12.7 Vietnam
13.5.12.8 The Philippines
13.5.12.9 Australia
13.5.12.10 New Zealand
13.5.12.11 Rest of APAC
13.6. Middle East & Africa Lentiviral Vectors Market
13.6.1 Key Market Trends, Growth Factors and Opportunities
13.6.2 Top Key Companies
13.6.3 Historic and Forecasted Market Size by Segments
13.6.4 Historic and Forecasted Market Size By Component
13.6.4.1 Lentiviral promoter
13.6.4.2 Lentiviral fusion tags
13.6.4.3 Lentivirus packaging systems and Other
13.6.5 Historic and Forecasted Market Size By Type
13.6.5.1 Product and Services
13.6.6 Historic and Forecasted Market Size By Generation
13.6.6.1 4th-generation
13.6.6.2 3rd-generation
13.6.6.3 2nd-generation and 1st-generation
13.6.7 Historic and Forecasted Market Size By Workflow
13.6.7.1 Upstream Processing And Downstream Processing
13.6.8 Historic and Forecasted Market Size By Delivery Method
13.6.8.1 In Vivo and Ex Vivo
13.6.9 Historic and Forecasted Market Size By Disease Indication
13.6.9.1 Cancer
13.6.9.2 Genetic Disorders
13.6.9.3 Infectious Diseases
13.6.9.4 Veterinary Disease And Other
13.6.10 Historic and Forecasted Market Size By Application
13.6.10.1 Gene Therapy And Vaccinology
13.6.11 Historic and Forecasted Market Size By End User
13.6.11.1 Biotechnology Companies
13.6.11.2 Pharmaceutical Companies
13.6.11.3 Contract Research Organizations
13.6.11.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
13.6.12 Historic and Forecast Market Size by Country
13.6.12.1 Turkiye
13.6.12.2 Bahrain
13.6.12.3 Kuwait
13.6.12.4 Saudi Arabia
13.6.12.5 Qatar
13.6.12.6 UAE
13.6.12.7 Israel
13.6.12.8 South Africa
13.7. South America Lentiviral Vectors Market
13.7.1 Key Market Trends, Growth Factors and Opportunities
13.7.2 Top Key Companies
13.7.3 Historic and Forecasted Market Size by Segments
13.7.4 Historic and Forecasted Market Size By Component
13.7.4.1 Lentiviral promoter
13.7.4.2 Lentiviral fusion tags
13.7.4.3 Lentivirus packaging systems and Other
13.7.5 Historic and Forecasted Market Size By Type
13.7.5.1 Product and Services
13.7.6 Historic and Forecasted Market Size By Generation
13.7.6.1 4th-generation
13.7.6.2 3rd-generation
13.7.6.3 2nd-generation and 1st-generation
13.7.7 Historic and Forecasted Market Size By Workflow
13.7.7.1 Upstream Processing And Downstream Processing
13.7.8 Historic and Forecasted Market Size By Delivery Method
13.7.8.1 In Vivo and Ex Vivo
13.7.9 Historic and Forecasted Market Size By Disease Indication
13.7.9.1 Cancer
13.7.9.2 Genetic Disorders
13.7.9.3 Infectious Diseases
13.7.9.4 Veterinary Disease And Other
13.7.10 Historic and Forecasted Market Size By Application
13.7.10.1 Gene Therapy And Vaccinology
13.7.11 Historic and Forecasted Market Size By End User
13.7.11.1 Biotechnology Companies
13.7.11.2 Pharmaceutical Companies
13.7.11.3 Contract Research Organizations
13.7.11.4 Contract Development and Manufacturing Organization (CDMO) and Academic/Research Institutes
13.7.12 Historic and Forecast Market Size by Country
13.7.12.1 Brazil
13.7.12.2 Argentina
13.7.12.3 Rest of SA
Chapter 14 Analyst Viewpoint and Conclusion
14.1 Recommendations and Concluding Analysis
14.2 Potential Market Strategies
Chapter 15 Research Methodology
15.1 Research Process
15.2 Primary Research
15.3 Secondary Research
|
Lentiviral Vectors Market |
|||
|
Base Year: |
2023 |
Forecast Period: |
2024-2032 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 290.3 Million |
|
Forecast Period 2024-32 CAGR: |
18.7% |
Market Size in 2032: |
USD 1358 Million |
|
Segments Covered: |
By Component |
|
|
|
By Type |
|
||
|
By Generation |
|
||
|
By Workflow |
|
||
|
By Delivery Method |
|
||
|
By Disease Indication |
|
||
|
By Application |
|
||
|
By End User |
|
||
|
By Region |
|
||
|
Key Market Drivers: |
|
||
|
Key Market Restraints: |
|
||
|
Key Opportunities: |
|
||
|
Companies Covered in the report: |
|
||













